Invertebrate Community Structure Along a Habitat-Patch Size Gradient Within a Bog Pool Complex
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
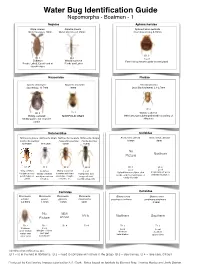
Water Bug ID Guide
Water Bug Identification Guide Nepomorpha - Boatmen - 1 Nepidae Aphelocheridae Nepa cinerea Ranatra linearis Aphelocheirus aestivalis Water Scorpion, 20mm Water Stick Insect, 35mm River Saucer bug, 8-10mm ID 1 ID 1 ID 1 Local Common Widely scattered Fast flowing streams under stones/gravel Ponds, Lakes, Canals and at Ponds and Lakes stream edges Naucoridae Pleidae Ilycoris cimicoides Naucoris maculata Plea minutissima Saucer bug, 13.5mm 10mm Least Backswimmer, 2.1-2.7mm ID 1 ID 1 ID 2 Widely scattered Widely scattered NORFOLK ONLY Often amongst submerged weed in a variety of Muddy ponds and stagnant stillwaters canals Notonectidae Corixidae Notonecta glauca Notonecta viridis Notonecta maculata Notonecta obliqua Arctocorisa gemari Arctocorisa carinata Common Backswimmer Peppered Backswimmer Pied Backswimmer 8.8mm 9mm 14-16mm 13-15mm 15mm 15mm No Northern Picture ID 2 ID 2 ID 2 ID 2 ID 3 ID 3 Local Local Very common Common Widely scattered Local Upland limestone lakes, dew In upland peat pools Ubiquitous in all Variety of waters In waters with hard Peat ponds, acid ponds, acid moorland lakes, or with little vegetation ponds, lakes or usually more base substrates, troughs, bog pools and sandy silt ponds canals rich sites concrete, etc. recently clay ponds Corixidae Corixidae Micronecta Micronecta Micronecta Micronecta Glaenocorisa Glaenocorisa scholtzi poweri griseola minutissima propinqua cavifrons propinqua propinqua 2-2.5mm 1.8mm 1.8mm 2mm 8.3mm No NEW RARE Northern Southern Picture ARIVAL ID 2 ID 2 ID 4 ID 4 ID 3 ID 3 Local Common Local Local Margins of rivers open shallow Northern Southern and quiet waters over upland lakes upland lakes silt or sand backwaters Identification difficulties are: ID 1 = id in the field in Northants. -

Variations on a Theme
HENRY JOUTSIJOKI Variations on a Theme The Classification of Benthic Macroinvertebrates ACADEMIC DISSERTATION To be presented, with the permission of the board of the School of Information Sciences of the University of Tampere, for public discussion in the Auditorium Pinni B 1100, Kanslerinrinne 1, Tampere, on November 9th, 2012, at 12 o’clock. UNIVERSITY OF TAMPERE ACADEMIC DISSERTATION University of Tampere School of Information Sciences Finland Copyright ©2012 Tampere University Press and the author Distribution Tel. +358 40 190 9800 Bookshop TAJU [email protected] P.O. Box 617 www.uta.fi/taju 33014 University of Tampere http://granum.uta.fi Finland Cover design by Mikko Reinikka Acta Universitatis Tamperensis 1777 Acta Electronica Universitatis Tamperensis 1251 ISBN 978-951-44-8952-5 (print) ISBN 978-951-44-8953-2 (pdf) ISSN-L 1455-1616 ISSN 1456-954X ISSN 1455-1616 http://acta.uta.fi Tampereen Yliopistopaino Oy – Juvenes Print Tampere 2012 Abstract This thesis focused on the classification of benthic macroinvertebrates by us- ing machine learning methods. Special emphasis was placed on multi-class extensions of Support Vector Machines (SVMs). Benthic macroinvertebrates are used in biomonitoring due to their properties to react to changes in water quality. The use of benthic macroinvertebrates in biomonitoring requires a large number of collected samples. Traditionally benthic macroinvertebrates are separated and identified manually one by one from samples collected by biologists. This, however, is a time-consuming and expensive approach. By the automation of the identification process time and money would be saved and more extensive biomonitoring would be possible. The aim of the thesis was to examine what classification method would be the most appro- priate for automated benthic macroinvertebrate classification. -

A Review of the Hemiptera of Great Britain: the Aquatic and Semi-Aquatic Bugs
Natural England Commissioned Report NECR188 A review of the Hemiptera of Great Britain: The Aquatic and Semi-aquatic Bugs Dipsocoromorpha, Gerromorpha, Leptopodomorpha & Nepomorpha Species Status No.24 First published 20 November 2015 www.gov.uk/natural -england Foreword Natural England commission a range of reports from external contractors to provide evidence and advice to assist us in delivering our duties. The views in this report are those of the authors and do not necessarily represent those of Natural England. Background Making good decisions to conserve species should primarily be based upon an objective process of determining the degree of threat to the survival of a species. The recognised international approach to undertaking this is by assigning the species to one of the IUCN threat categories. This report was commissioned to update the national status of aquatic and semi-aquatic bugs using IUCN methodology for assessing threat. It covers all species of aquatic and semi-aquatic bugs (Heteroptera) in Great Britain, identifying those that are rare and/or under threat as well as non-threatened and non-native species. Reviews for other invertebrate groups will follow. Natural England Project Manager - Jon Webb, [email protected] Contractor - A.A. Cook (author) Keywords - invertebrates, red list, IUCN, status reviews, Heteroptera, aquatic bugs, shore bugs, IUCN threat categories, GB rarity status Further information This report can be downloaded from the Natural England website: www.gov.uk/government/organisations/natural-england. For information on Natural England publications contact the Natural England Enquiry Service on 0845 600 3078 or e-mail [email protected]. -

Het News Issue 3
Issue 3 Spring 2004 Het News nd 2 Series Newsletter of the Heteroptera Recording Schemes Editorial: There is a Dutch flavour to this issue which we hope will be of interest. After all, The Netherlands is not very far as the bug flies and with a following wind there could easily be immigrants reaching our shores at any time. We have also introduced an Archive section, for historical articles, to appear when space allows. As always we are very grateful to all the providers of material for this issue and, for the next issue, look forward to hearing about your 2004 (& 2003) exploits, exciting finds, regional news, innovative gadgets etc. Sheila Brooke 18 Park Hill Toddington Dunstable Beds LU5 6AW [email protected] Bernard Nau 15 Park Hill Toddington Dunstable Beds LU5 6AW [email protected] Contents Editorial .................................................................... 1 Forthcoming & recent events ................................. 7 Dutch Bug Atlas....................................................... 1 Checklist of British water bugs .............................. 8 Recent changes in the Dutch Heteroptera............. 2 The Lygus situation............................................... 11 Uncommon Heteroptera from S. England ............. 5 Web Focus.............................................................. 12 News from the Regions ........................................... 6 From the Archives ................................................. 12 Gadget corner – Bug Mailer..................................... -

The Freshwater Bugs (Hemiptera: Heteroptera) of Cheshire
The freshwater bugs (Hemiptera: Heteroptera) of Cheshire Jonathan Guest Germany. The late Dr. Alan Savage School of Life Sciences, Keele University, STS 5BG. Dr. Ian Wallace Entomology Section, Liverpool Museum, William Brown St., Liverpool, L3 8EN.* ______________________________ *address for correspondence_____________________ Introduction Cheshire is predominantly a lowland county. It has a very large number of ponds, internationally important habitats in the form of meres and mosses, intriguing inland saline waters associated with the salt industry and two major estuaries. Upland water bodies are restricted to the far east of the county. Eleven families of British Heteroptera are grouped as aquatic bugs with 64 species in 23 genera; Cheshire is both well-recorded and well-represented with 10 families, and 48 species in 21 genera. By stark contrast, the record coverage maps in Huxley (2003) show clearly that Lancashire, away from the coast, and especially south Lancashire, is very poorly recorded. Water bugs are very familiar freshwater insects, with most species being easy to catch in a pond-net; a few are best sought by pressing marginal vegetation underwater, and one lives amongst Sphagnum moss. They can be preserved in isopropyl alcohol, or a 70% ethyl alcohol solution - the traditional medium used by freshwater biologists, or pinned, or even carded - but important identification features are found on the underside in several genera. Identification is straightforward and satisfying using Savage (1989) for adults; for immatures of the large family Corixidae see Savage (1999) and for young pondskaters there is Brinkhurst (1959). Information sources Britten (1930) provided the first check-list for Cheshire water bugs, which was updated by Massee (1955) and Judd (1990). -

1 Environmental Factors Are Primary Determinants of Different Facets of Pond Macroinvertebrate
1 Environmental factors are primary determinants of different facets of pond macroinvertebrate 2 alpha and beta diversity in a human-modified landscape 3 Running title: Pond biodiversity in a human-modified landscape 4 5 Abstract 6 Understanding the spatial patterns and environmental drivers of freshwater diversity and community 7 structure is a key challenge in biogeography and conservation biology. However, previous studies 8 have focussed primarily on taxonomic diversity and have largely ignored the phylogenetic and 9 functional facets resulting in an incomplete understanding of the community assembly. Here, we 10 examine the influence of local environmental, hydrological proximity effects, land-use type and 11 spatial structuring on taxonomic, functional and phylogenetic (using taxonomic relatedness as a 12 proxy) alpha and beta diversity (including the turnover and nestedness-resultant components) of pond 13 macroinvertebrate communities. Ninety-five ponds across urban and non-urban land-uses in 14 Leicestershire, UK were examined. Functional and phylogenetic alpha diversity were negatively 15 correlated with species richness. At the alpha scale, functional diversity and taxonomic richness were 16 primarily determined by local environmental factors while phylogenetic alpha diversity was driven by 17 spatial factors. Compositional variation (beta diversity) of the different facets and components of 18 functional and phylogenetic diversity were largely determined by local environmental variables. Pond 19 surface area, dry phase length and macrophyte cover were consistently important predictors of the 20 different facets and components of alpha and beta diversity. Our results suggest that pond 21 management activities aimed at improving biodiversity should focus on improving and/or restoring 22 local environmental conditions. -

Survey of Aquatic Macro-Invertebrates in New Dykes and a Pond at Paull, East Yorkshire
Survey of aquatic macro-invertebrates in new dykes and a pond at Paull, East Yorkshire Summary Realignment of the Humber bank between Paull and Thorgumbald resulted in loss of a large borrow pit situated behind the original embankment which was known to support a rich assemblage of water beetles and other aquatic insects. At the same time, new aquatic habitats have been provided in the form of extensive dykes and a pond behind the new embankments. In July 2003, vegetation was transferred from the former borrow pit to the new water bodies and an attempt was also made to translocate invertebrates. A survey of aquatic macroinvertebrates in the new water bodies was undertaken in April 2004, both to judge the effectiveness of the translocation work and to assess the overall fauna. The new water bodies support several scarce water beetles. These include opportunistic colonists of raw ponds such as Hygrotus nigrolineatus and Scarodytes halensis as well as specialists of coastal / brackish water habitats including Haliplus apicalis, Agabus conspersus and Enochrus bicolor. Some of these species may be of ephemeral occurrence but others will hopefully have established resident populations. None of the specialist brackish water Corixid bugs were found but these had not been present in the former borrow pit. Caddis flies were represented by two larval taxa, one of which is typical of ion-rich waters. The brackish water amphipod Gammarus zaddachi is abundant. Most other aquatic invertebrates were ubiquitous freshwater or wide tolerance species. It is difficult to evaluate the efficacy of translocation of vegetation and invertebrates from the former borrow pit to the new water bodies. -
Callicorixa Praeusta (Fieber) and Callicorixa Wollastoni (Douglas & Scott) (Hemiptera Heteroptera: Corixidae) Alan Savage and Emma Swift
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by FBA Journal System (Freshwater Biological... IDENTIFICATION OF ADULT CORIXIDS 25 THE IDENTIFICATION OF BRITISH ADULT SPECIMENS OF SIGARA LATERALIS (LEACH), SIGARA CONCINNA (FIEBER), CALLICORIXA PRAEUSTA (FIEBER) AND CALLICORIXA WOLLASTONI (DOUGLAS & SCOTT) (HEMIPTERA HETEROPTERA: CORIXIDAE) ALAN SAVAGE AND EMMA SWIFT (Dr A. A. Savage and E. J. Swift, Department of Biological Sciences, Keele University, Keele, Staffordshire ST5 5BG, England.) Introduction The four species named in the title of this article all have dark, virtually black, markings on the tarsi and/or claws of the posterior legs. Indeed, typical specimens are most easily identified by the different positions and shapes of these markings which, therefore, have been used as diagnostic features in keys to Corixidae (Saunders 1892; Butler 1923; Macan 1939, 1956, 1965; Southwood & Leston 1959; Savage 1989, 1990). However, it has long been recognised that specimens with atypical markings are sometimes found which, together with newly emerged (teneral) adults, increase the difficulty of accurate identification. In general, the dark markings on mature adults have totally reliable diagnostic features only in S. lateralis. The other three species, S. concinna, C. wollastoni and C. praeusta, form an increasingly variable series with the possibility of overlap between all three species. Furthermore, a degree of variation in the perception of a species in the genus Callicorixa has exacerbated the problems of identification. The aim of this communication is to briefly review nomenclature in the genus Callicorixa, describe the variation in the dark markings on the posterior legs of all four species, describe alternative diagnostic features, and provide a key to identification based on these alternative features. -

Macroinvertebrate Classification Diagnostic Tool Development
Final Report Project WFD60 Macroinvertebrate classification diagnostic tool development August 2007 SNIFFER WFD60: Macroinvertebrate Classification Diagnostic Tool August 2007 © SNIFFER 2007 All rights reserved. No part of this document may be reproduced, stored in a retrieval system or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise without the prior permission of SNIFFER. The views expressed in this document are not necessarily those of SNIFFER. Its members, servants or agents accept no liability whatsoever for any loss or damage arising from the interpretation or use of the information, or reliance upon views contained herein. Dissemination status Unrestricted Use of this report The development of UK-wide classification methods and environmental standards that aim to meet the requirements of the Water Framework Directive (WFD) is being sponsored by UK Technical Advisory Group (UKTAG) for WFD on behalf its member and partners. This technical document has been developed through a collaborative project, managed and facilitated by SNIFFER and has involved the members and partners of UKTAG. It provides background information to support the ongoing development of the standards and classification methods. Whilst this document is considered to represent the best available scientific information and expert opinion available at the stage of completion of the report, it does not necessarily represent the final or policy positions of UKTAG or any of its partner agencies. Project funders SNIFFER, -

Development of a Novel Invertebrate Indexing Tool for the Determination of Salinity in Aquatic Inland Drainage Channels
DEVELOPMENT OF A NOVEL INVERTEBRATE INDEXING TOOL FOR THE DETERMINATION OF SALINITY IN AQUATIC INLAND DRAINAGE CHANNELS Alexander G. G. Pickwell A thesis submitted in partial fulfilment of the requirements of the University of Lincoln for the degree of Master of Philosophy in Biology This research programme was carried out in collaboration with the Environment Agency August 2012 Page i Certificate of Originality This is to certify that I am responsible for the work submitted in this thesis, that the original work is my own, except as specified in the acknowledgements and in references, and that neither the thesis nor the original work contained therein has been previously submitted to any institution for the award of a degree. Signature: Name: Alexander G. G. Pickwell Date: 31st August 2012 Page i Abstract Salinisation of freshwater habitats is an issue with global implications that can have serious detrimental effects on the environment resulting in an overall loss in biodiversity. Whilst increases in salinity can occur naturally, such anthropogenic actions as the disposal of industrial and urban effluents and the disturbance of natural hydrological cycles can also result in the salinisation of freshwater habitats. The Water Framework Directive (WFD) requires Member States to restore all freshwater habitats to “good ecological status” and to prevent any further deterioration. Macro-invertebrates are widely used as indicators of river condition for a wide range of reasons and have been designated a key biological element in the assessment of aquatic habitats by the WFD. A review of the available literature, however, found no macro- invertebrate-based biotic indices have been developed for the detection and determination of salinity increases in freshwater habitats that are suitable for application in the United Kingdom for the purposes of the WFD. -
The Biodiversity and Metabolism of Peatland Pools
THE BIODIVERSITY AND METABOLISM OF PEATLAND POOLS JEAN MARIA BEADLE Submitted in accordance with the requirements for the degree of Doctor of Philosophy The University of Leeds School of Geography September 2015 The candidate confirms that the work submitted is her own, except where work which has formed part of jointly authored publications has been included. The contribution of the candidate and the other authors to this work has been explicitly indicated below. The candidate confirms that appropriate credit has been given within the thesis where reference has been made to the work of others. Chapter 2 of this thesis is based on: Beadle Jeannie M, Brown Lee E, Holden Joseph (2015). Biodiversity and ecosystem functioning in natural bog pools and those created by rewetting schemes. WIREs Water, 2: 65-84. doi: 10.1002/wat2.1063. The paper was written by the candidate but review comments from both co-authors were included in the submitted version. This copy has been supplied on the understanding that it is copyright material and that no quotation from the thesis may be published without proper acknowledgement. © 2015 The University of Leeds and Jean Maria Beadle Acknowledgements I am especially thankful to my supervisors, Dr. Lee Brown and Professor Joseph Holden, for their invaluable guidance, encouragement and support with all aspects of my research. I am also extremely grateful to Simon Wightman and Gareth Edwards, my supervisors from the Royal Society for the Protection of Birds (RSPB), and to Steve Garnett from the RSPB for all his help with site selection and access at Geltsdale RSPB Reserve. -
Further Development of River Invertebrate Classification Tool
Final Report Project WFD100 Further Development of River Invertebrate Classification Tool September/2010 © SNIFFER 2010 All rights reserved. No part of this document may be reproduced, stored in a retrieval system or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise without the prior permission of SNIFFER. The views expressed in this document are not necessarily those of SNIFFER. Its members, servants or agents accept no liability whatsoever for any loss or damage arising from the interpretation or use of the information, or reliance upon views contained herein. Dissemination status Unrestricted Project funders Environment Agency Northern Ireland Environment Agency Scottish Environment Protection Agency Whilst this document is considered to represent the best available scientific information and expert opinion available at the stage of completion of the report, it does not necessarily represent the final or policy positions of the project funders. Research contractor This document was produced by the Centre for Ecology & Hydrology (main contractor) and the Freshwater Biological Association (sub-contractor): John Davy-Bowker*, Sean Arnott‡, Rebecca Close†, Mike Dobson†, Michael Dunbar‡, Gabriela Jofre*, Diana Morton*, John Murphy∆, William Wareham‡, Stephanie Smith* & Vanya Gordon* *The Freshwater Biological Association, River Laboratory, East Stoke, Wareham, Dorset, BH20 6BB, United Kingdom †The Freshwater Biological Association, The Ferry Landing, Far Sawrey, Ambleside, Cumbria, LA22