Macroinvertebrate Classification Diagnostic Tool Development
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spezialpraktikum Aquatische Und Semiaquatische Heteroptera SS 2005
Spezialpraktikum Aquatische und Semiaquatische Heteroptera SS 2005 Hinweise zum Bestimmungsschlüssel Das Skriptum ist für den Privatgebrauch bestimmt. Alle Angaben wurden nach bestem Wissen überprüft, Fehler sind aber nicht auszuschließen. Für Anmerkungen und Korrekturen bin ich sehr dankbar. Alle Abbildungen wurden der Literatur entnommen (siehe Literaturverzeichnis) und sind nicht Maßstabsgetreu, d.h. die Größenverhältnisse sind nicht korrekt wiedergegeben. Die Gesamtverbreitungsangaben wurden aus Aukema & Rieger (1995) entnommen und sind daher nicht aktuell. Sie geben aber einen guten Eindruck von der ungefähren Verbreitung der Arten. Die verwendeten zoogeographischen Angaben sind vorläufig und rein deskriptiv zu verstehen. Die Angabe der Bundesländervorkommen in Österreich erfolgt ohne Anspruch auf Vollständigkeit (Rabitsch unveröff., Stand 2005) Wolfgang Rabitsch Wien, Juni 2005 Version 1.1 (Juli 2005) Bestimmungsschlüssel der GERROMORPHA Österreichs Bestimmungsschlüssel der GERROMORPHA Österreichs (verändert nach Andersen 1993, 1995, 1996, Savage 1989, u.a.) Familien (Abb. 1) 1 Kopf mindestens doppelt so lang wie breit, Augen deutlich vom Vorderrand des Prothorax entfernt Hydrometridae - Kopf weniger als doppelt so lang wie breit, Augen berühren fast den Vorderrand des Prothorax 2 2 Coxae inserieren in der Körpermitte, Antennen 4-gliedrig, meist apter Mesoveliidae - Coxae inserieren lateral am Thorax 3 3 Antennen scheinbar 5-gliedrig, Segment 3-5 dünner als Segment 1-2, Ocellen vorhanden Hebridae - Antennen 4-gliedrig, alle Segmente -

Water Beetles
Ireland Red List No. 1 Water beetles Ireland Red List No. 1: Water beetles G.N. Foster1, B.H. Nelson2 & Á. O Connor3 1 3 Eglinton Terrace, Ayr KA7 1JJ 2 Department of Natural Sciences, National Museums Northern Ireland 3 National Parks & Wildlife Service, Department of Environment, Heritage & Local Government Citation: Foster, G. N., Nelson, B. H. & O Connor, Á. (2009) Ireland Red List No. 1 – Water beetles. National Parks and Wildlife Service, Department of Environment, Heritage and Local Government, Dublin, Ireland. Cover images from top: Dryops similaris (© Roy Anderson); Gyrinus urinator, Hygrotus decoratus, Berosus signaticollis & Platambus maculatus (all © Jonty Denton) Ireland Red List Series Editors: N. Kingston & F. Marnell © National Parks and Wildlife Service 2009 ISSN 2009‐2016 Red list of Irish Water beetles 2009 ____________________________ CONTENTS ACKNOWLEDGEMENTS .................................................................................................................................... 1 EXECUTIVE SUMMARY...................................................................................................................................... 2 INTRODUCTION................................................................................................................................................ 3 NOMENCLATURE AND THE IRISH CHECKLIST................................................................................................ 3 COVERAGE ....................................................................................................................................................... -

Crossness Sewage Treatment Works Nature Reserve & Southern Marsh Aquatic Invertebrate Survey
Commissioned by Thames Water Utilities Limited Clearwater Court Vastern Road Reading RG1 8DB CROSSNESS SEWAGE TREATMENT WORKS NATURE RESERVE & SOUTHERN MARSH AQUATIC INVERTEBRATE SURVEY Report number: CPA18054 JULY 2019 Prepared by Colin Plant Associates (UK) Consultant Entomologists 30a Alexandra Rd London N8 0PP 1 1 INTRODUCTION, BACKGROUND AND METHODOLOGY 1.1 Introduction and background 1.1.1 On 30th May 2018 Colin Plant Associates (UK) were commissioned by Biodiversity Team Manager, Karen Sutton on behalf of Thames Water Utilities Ltd. to undertake aquatic invertebrate sampling at Crossness Sewage Treatment Works on Erith Marshes, Kent. This survey was to mirror the locations and methodology of a previous survey undertaken during autumn 2016 and spring 2017. Colin Plant Associates also undertook the aquatic invertebrate sampling of this previous survey. 1.1.2 The 2016-17 aquatic survey was commissioned with the primary objective of establishing a baseline aquatic invertebrate species inventory and to determine the quality of the aquatic habitats present across both the Nature Reserve and Southern Marsh areas of the Crossness Sewage Treatment Works. The surveyors were asked to sample at twenty-four, pre-selected sample station locations, twelve in each area. Aquatic Coleoptera and Heteroptera (beetles and true bugs) were selected as target groups. A report of the previous survey was submitted in Sept 2017 (Plant 2017). 1.1.3 During December 2017 a large-scale pollution event took place and untreated sewage escaped into a section of the Crossness Nature Reserve. The primary point of egress was Nature Reserve Sample Station 1 (NR1) though because of the connectivity of much of the waterbody network on the marsh other areas were affected. -

Anisus Vorticulus (Troschel 1834) (Gastropoda: Planorbidae) in Northeast Germany
JOURNAL OF CONCHOLOGY (2013), VOL.41, NO.3 389 SOME ECOLOGICAL PECULIARITIES OF ANISUS VORTICULUS (TROSCHEL 1834) (GASTROPODA: PLANORBIDAE) IN NORTHEAST GERMANY MICHAEL L. ZETTLER Leibniz Institute for Baltic Sea Research Warnemünde, Seestr. 15, D-18119 Rostock, Germany Abstract During the EU Habitats Directive monitoring between 2008 and 2010 the ecological requirements of the gastropod species Anisus vorticulus (Troschel 1834) were investigated in 24 different waterbodies of northeast Germany. 117 sampling units were analyzed quantitatively. 45 of these units contained living individuals of the target species in abundances between 4 and 616 individuals m-2. More than 25.300 living individuals of accompanying freshwater mollusc species and about 9.400 empty shells were counted and determined to the species level. Altogether 47 species were identified. The benefit of enhanced knowledge on the ecological requirements was gained due to the wide range and high number of sampled habitats with both obviously convenient and inconvenient living conditions for A. vorticulus. In northeast Germany the amphibian zones of sheltered mesotrophic lake shores, swampy (lime) fens and peat holes which are sun exposed and have populations of any Chara species belong to the optimal, continuously and densely colonized biotopes. The cluster analysis emphasized that A. vorticulus was associated with a typical species composition, which can be named as “Anisus-vorticulus-community”. In compliance with that both the frequency of combined occurrence of species and their similarity in relative abundance are important. The following species belong to the “Anisus-vorticulus-community” in northeast Germany: Pisidium obtusale, Pisidium milium, Pisidium pseudosphaerium, Bithynia leachii, Stagnicola palustris, Valvata cristata, Bathyomphalus contortus, Bithynia tentaculata, Anisus vortex, Hippeutis complanatus, Gyraulus crista, Physa fontinalis, Segmentina nitida and Anisus vorticulus. -

Position Specificity in the Genus Coreomyces (Laboulbeniomycetes, Ascomycota)
VOLUME 1 JUNE 2018 Fungal Systematics and Evolution PAGES 217–228 doi.org/10.3114/fuse.2018.01.09 Position specificity in the genus Coreomyces (Laboulbeniomycetes, Ascomycota) H. Sundberg1*, Å. Kruys2, J. Bergsten3, S. Ekman2 1Systematic Biology, Department of Organismal Biology, Evolutionary Biology Centre, Uppsala University, Uppsala, Sweden 2Museum of Evolution, Uppsala University, Uppsala, Sweden 3Department of Zoology, Swedish Museum of Natural History, Stockholm, Sweden *Corresponding author: [email protected] Key words: Abstract: To study position specificity in the insect-parasitic fungal genus Coreomyces (Laboulbeniaceae, Laboulbeniales), Corixidae we sampled corixid hosts (Corixidae, Heteroptera) in southern Scandinavia. We detected Coreomyces thalli in five different DNA positions on the hosts. Thalli from the various positions grouped in four distinct clusters in the resulting gene trees, distinctly Fungi so in the ITS and LSU of the nuclear ribosomal DNA, less so in the SSU of the nuclear ribosomal DNA and the mitochondrial host-specificity ribosomal DNA. Thalli from the left side of abdomen grouped in a single cluster, and so did thalli from the ventral right side. insect Thalli in the mid-ventral position turned out to be a mix of three clades, while thalli growing dorsally grouped with thalli from phylogeny the left and right abdominal clades. The mid-ventral and dorsal positions were found in male hosts only. The position on the left hemelytron was shared by members from two sister clades. Statistical analyses demonstrate a significant positive correlation between clade and position on the host, but also a weak correlation between host sex and clade membership. These results indicate that sex-of-host specificity may be a non-existent extreme in a continuum, where instead weak preferences for one host sex may turn out to be frequent. -

Freshwater Snail Guide
A Beginner’s Guide to Freshwater Snails of Central Scotland Freshwater snails are a diverse and fascinating group of molluscs found in ponds, lochs, canals, rivers and watery ditches all across the globe. This guide is a simple introduction to some of the most commonly encountered and easily identifiable freshwater snail species in Central Scotland. How do I look for freshwater snails? 1) Choose your spot: anywhere with water – your garden pond, a nearby river, canal or loch – is likely to have snails. 2) Grab a pond net (buy online or make your own by attaching a sieve to the end of an old broom or mop), a white tray or tub and a hand-lens or magnifier. 3) Fill the tray with water from your chosen habitat. Sweep the net around in the water, making sure to get among any vegetation (don’t go too near the bottom or you’ll get a net full of mud), empty your net into the water-filled tray and identify what you have found! Snail shell features Spire Whorls Some snail species have a hard plate called an ‘operculum’ Height which they Mouth (Aperture) use to seal the mouth of the shell when they are inside Height Pointed shell Flat shell Width Width Pond Snails (Lymnaeidae) Variable in size. Mouth always on right-hand side, shells usually long and pointed. Great Pond Snail Common Pond Snail Lymnaea stagnalis Radix balthica Largest pond snail. Common in ponds Fairly rounded and ’fat’. Common in weedy lakes, canals and sometimes slow river still waters. pools. -

Transactions Dumfriesshire and Galloway Natural History Antiquarian Society
Transactions of the Dumfriesshire and Galloway Natural History and Antiquarian Society LXXXIV 2010 Transactions of the Dumfriesshire and Galloway Natural History and Antiquarian Society FOUNDED 20th NOVEMBER, 1862 THIRD SERIES VOLUME LXXXIV Editors: ELAINE KENNEDY FRANCIS TOOLIS ISSN 0141-1292 2010 DUMFRIES Published by the Council of the Society Office-Bearers 2009-2010 and Fellows of the Society President Morag Williams MA Vice Presidents Dr A Terry, Mr J L Williams, Mrs J Brann and Mr R Copeland Fellows of the Society Mr J Banks BSc, Mr A D Anderson BSc, Mr J Chinnock, Mr J H D Gair MA, Dr J B Wilson MD, Mr K H Dobie, Mrs E Toolis and Dr D F Devereux Mr L J Masters and Mr R H McEwen — appointed under Rule 10 Hon. Secretary John L Williams, Merkland, Kirkmahoe, Dumfries DG1 1SY Hon. Membership Secretary Miss H Barrington, 30 Noblehill Avenue, Dumfries DG1 3HR Hon. Treasurer Mr L Murray, 24 Corberry Park, Dumfries DG2 7NG Hon. Librarian Mr R Coleman, 2 Loreburn Park, Dumfries DG1 1LS Hon. Editors Mr James Williams (until November 2009) Elaine Kennedy, Nether Carruchan, Troqueer, Dumfries DG2 8LY (from January 2010) Dr F Toolis, 25 Dalbeattie Road, Dumfries DG2 7PF Dr J Foster (Webmaster), 21 Maxwell Street, Dumfries DG2 7AP Hon. Syllabus Convener Mrs E Toolis, 25 Dalbeattie Road, Dumfries DG2 7PF Hon. Curators Joanne Turner and Siobhan Ratchford Hon. Outings Organisers Mr J Copland and Mr A Gair Ordinary Members Mrs P G Williams, Mr D Rose, Mrs C Iglehart, Mr A Pallister, Mrs A Weighill, Mrs S Honey CONTENTS Rosa Gigantea - George Watt, including ‘On the Trail of Two Knights’ by Girija Viraraghavan by Morag Williams ........................................................... -

Speeding up the Snail's Pace Bird
PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/93702 Please be advised that this information was generated on 2021-10-07 and may be subject to change. SPEEDING UP THE SNAIL’S PACE Bird-mediated dispersal of aquatic organisms Casper H.A. van Leeuwen Speeding up the snail’s pace Bird-mediated dispersal of aquatic organisms The work in this thesis was conducted at the Netherlands Institute of Ecology (NIOO-KNAW) and Radboud University Nijmegen, cooperating within the Centre for Wetland Ecology. This thesis should be cited as: Van Leeuwen, C.H.A. (2012) Speeding up the snail’s pace: bird-mediated dispersal of aquatic organisms. PhD thesis, Radboud University Nijmegen, Nijmegen, The Netherlands ISBN: 978-90-6464-566-2 Printed by Ponsen & Looijen, Ede, The Netherlands Speeding up the snail’s pace Bird-mediated dispersal of aquatic organisms PROEFSCHRIFT ter verkrijging van de graad van doctor aan de Radboud Universiteit Nijmegen op het gezag van de rector magnificus prof. mr. S.C.J.J. Kortmann, volgens besluit van het College van Decanen in het openbaar te verdedigen op woensdag 27 juni 2012 om 13.00 uur precies door Casper Hendrik Abram van Leeuwen geboren op 18 september 1983 te Odijk Promotoren: Prof. dr. Jan van Groenendael Prof. dr. Marcel Klaassen (Universiteit Utrecht) Copromotor: Dr. Gerard van der Velde Manuscriptcommissie: Prof. dr. Hans de Kroon Dr. Gerhard Cadée (Koninklijk NIOZ) Prof. dr. Edmund Gittenberger (Universiteit Leiden) Prof. -

A Manual for the Survey and Evaluation of the Aquatic Plant and Invertebrate Assemblages of Grazing Marsh Ditch Systems
A manual for the survey and evaluation of the aquatic plant and invertebrate assemblages of grazing marsh ditch systems Version 6 Margaret Palmer Martin Drake Nick Stewart May 2013 Contents Page Summary 3 1. Introduction 4 2. A standard method for the field survey of ditch flora 5 2.1 Field survey procedure 5 2.2 Access and licenses 6 2.3 Guidance for completing the recording form 6 Field recording form for ditch vegetation survey 10 3. A standard method for the field survey of aquatic macro- invertebrates in ditches 12 3.1 Number of ditches to be surveyed 12 3.2 Timing of survey 12 3.3 Access and licences 12 3.4 Equipment 13 3.5 Sampling procedure 13 3.6 Taxonomic groups to be recorded 15 3.7 Recording in the field 17 3.8 Laboratory procedure 17 Field recording form for ditch invertebrate survey 18 4. A system for the evaluation and ranking of the aquatic plant and macro-invertebrate assemblages of grazing marsh ditches 19 4.1 Background 19 4.2 Species check lists 19 4.3 Salinity tolerance 20 4.4 Species conservation status categories 21 4.5 The scoring system 23 4.6 Applying the scoring system 26 4.7 Testing the scoring system 28 4.8 Conclusion 30 Table 1 Check list and scoring system for target native aquatic plants of ditches in England and Wales 31 Table 2 Check list and scoring system for target native aquatic invertebrates of grazing marsh ditches in England and Wales 40 Table 3 Some common plants of ditch banks that indicate salinity 50 Table 4 Aquatic vascular plants used as indicators of good habitat quality 51 Table 5a Introduced aquatic vascular plants 53 Table 5a Introduced aquatic invertebrates 54 Figure 1 Map of Environment Agency regions 55 5. -

SER. B VOL. 28 NO. 1 Norwegian Journal of Entomology
"'T ... 1981 SER. B VOL. 28 NO. 1 Norwegian Journal of Entomology PUBLISHED BY ORSK ZOOLOGISK TIDSSKRIFTSE TRAL OSW Fauna norvegica Ser. B Norwegian Journal of Entomology Norsk Entomologisk Forenings tidsskrift Appears with one volume (two issues) annually norske biblioteker. Andre ma betale kr. 55, -. Disse Utkommer med to hefter pr. af. innbetalinger sendes til NZT, Zoologisk museum, Editor-in-Chief (Ansvarlig redaktor) Sarsgt. I, Oslo 5. Ole A. S<ether, Museum of Zoology, Museplass 3, Postgiro 2 3483 65. 5014 Bergen/Univ. Editorial Committee (Redaksjonskomite) FAUNA NORVEGICA B publishes original new in Arne Nilssen, Zoological Dept., Troms0 Museum, formation generally relevant to Norwegian entomo N-9000 Troms0, John O. Solem, DKNVS Museet, logy. The journal emphasizes papers which are main Erling Skakkes gt. 47B, N-7000 Trondheim, Albert ly faunistical or zoogeographical in scope or con Lillehammer. Zoological Museum, Sars gt. I, tent, including checklists, faunal lists, type catalogues Oslo 5. and regional keys. Submissions must not have been previously published or copyrighted and must not be Subscription published subsequently except in abstract form or by Members of Norw.Ent.Soc. will recieve the journal written consent of the Editor-in-Chief. free. Membership fee N.kr. 50,- should be paid to the Treasurer of NEF: Use Hofsvang, Brattvollveien NORSK ENTOMOWGISK FORtNING 107, Oslo 11. Postgiro 5 44 09 20. Questions about ser sin oppgave i a fremme det entomologiske stu membership should be directed to the Secretary of dium i Norge, og danne et bindeledd melJom de in NEF. Trond Hofsvang, P.O. Box 70, N-1432 As teresserte. -
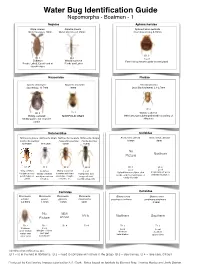
Water Bug ID Guide
Water Bug Identification Guide Nepomorpha - Boatmen - 1 Nepidae Aphelocheridae Nepa cinerea Ranatra linearis Aphelocheirus aestivalis Water Scorpion, 20mm Water Stick Insect, 35mm River Saucer bug, 8-10mm ID 1 ID 1 ID 1 Local Common Widely scattered Fast flowing streams under stones/gravel Ponds, Lakes, Canals and at Ponds and Lakes stream edges Naucoridae Pleidae Ilycoris cimicoides Naucoris maculata Plea minutissima Saucer bug, 13.5mm 10mm Least Backswimmer, 2.1-2.7mm ID 1 ID 1 ID 2 Widely scattered Widely scattered NORFOLK ONLY Often amongst submerged weed in a variety of Muddy ponds and stagnant stillwaters canals Notonectidae Corixidae Notonecta glauca Notonecta viridis Notonecta maculata Notonecta obliqua Arctocorisa gemari Arctocorisa carinata Common Backswimmer Peppered Backswimmer Pied Backswimmer 8.8mm 9mm 14-16mm 13-15mm 15mm 15mm No Northern Picture ID 2 ID 2 ID 2 ID 2 ID 3 ID 3 Local Local Very common Common Widely scattered Local Upland limestone lakes, dew In upland peat pools Ubiquitous in all Variety of waters In waters with hard Peat ponds, acid ponds, acid moorland lakes, or with little vegetation ponds, lakes or usually more base substrates, troughs, bog pools and sandy silt ponds canals rich sites concrete, etc. recently clay ponds Corixidae Corixidae Micronecta Micronecta Micronecta Micronecta Glaenocorisa Glaenocorisa scholtzi poweri griseola minutissima propinqua cavifrons propinqua propinqua 2-2.5mm 1.8mm 1.8mm 2mm 8.3mm No NEW RARE Northern Southern Picture ARIVAL ID 2 ID 2 ID 4 ID 4 ID 3 ID 3 Local Common Local Local Margins of rivers open shallow Northern Southern and quiet waters over upland lakes upland lakes silt or sand backwaters Identification difficulties are: ID 1 = id in the field in Northants. -

Variations on a Theme
HENRY JOUTSIJOKI Variations on a Theme The Classification of Benthic Macroinvertebrates ACADEMIC DISSERTATION To be presented, with the permission of the board of the School of Information Sciences of the University of Tampere, for public discussion in the Auditorium Pinni B 1100, Kanslerinrinne 1, Tampere, on November 9th, 2012, at 12 o’clock. UNIVERSITY OF TAMPERE ACADEMIC DISSERTATION University of Tampere School of Information Sciences Finland Copyright ©2012 Tampere University Press and the author Distribution Tel. +358 40 190 9800 Bookshop TAJU [email protected] P.O. Box 617 www.uta.fi/taju 33014 University of Tampere http://granum.uta.fi Finland Cover design by Mikko Reinikka Acta Universitatis Tamperensis 1777 Acta Electronica Universitatis Tamperensis 1251 ISBN 978-951-44-8952-5 (print) ISBN 978-951-44-8953-2 (pdf) ISSN-L 1455-1616 ISSN 1456-954X ISSN 1455-1616 http://acta.uta.fi Tampereen Yliopistopaino Oy – Juvenes Print Tampere 2012 Abstract This thesis focused on the classification of benthic macroinvertebrates by us- ing machine learning methods. Special emphasis was placed on multi-class extensions of Support Vector Machines (SVMs). Benthic macroinvertebrates are used in biomonitoring due to their properties to react to changes in water quality. The use of benthic macroinvertebrates in biomonitoring requires a large number of collected samples. Traditionally benthic macroinvertebrates are separated and identified manually one by one from samples collected by biologists. This, however, is a time-consuming and expensive approach. By the automation of the identification process time and money would be saved and more extensive biomonitoring would be possible. The aim of the thesis was to examine what classification method would be the most appro- priate for automated benthic macroinvertebrate classification.