Survey of Aquatic Macro-Invertebrates in New Dykes and a Pond at Paull, East Yorkshire
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Water Beetles
Ireland Red List No. 1 Water beetles Ireland Red List No. 1: Water beetles G.N. Foster1, B.H. Nelson2 & Á. O Connor3 1 3 Eglinton Terrace, Ayr KA7 1JJ 2 Department of Natural Sciences, National Museums Northern Ireland 3 National Parks & Wildlife Service, Department of Environment, Heritage & Local Government Citation: Foster, G. N., Nelson, B. H. & O Connor, Á. (2009) Ireland Red List No. 1 – Water beetles. National Parks and Wildlife Service, Department of Environment, Heritage and Local Government, Dublin, Ireland. Cover images from top: Dryops similaris (© Roy Anderson); Gyrinus urinator, Hygrotus decoratus, Berosus signaticollis & Platambus maculatus (all © Jonty Denton) Ireland Red List Series Editors: N. Kingston & F. Marnell © National Parks and Wildlife Service 2009 ISSN 2009‐2016 Red list of Irish Water beetles 2009 ____________________________ CONTENTS ACKNOWLEDGEMENTS .................................................................................................................................... 1 EXECUTIVE SUMMARY...................................................................................................................................... 2 INTRODUCTION................................................................................................................................................ 3 NOMENCLATURE AND THE IRISH CHECKLIST................................................................................................ 3 COVERAGE ....................................................................................................................................................... -
Water Measurer | Buglife Page 1 of 5
Water measurer | Buglife Page 1 of 5 Join Buglife Donate Volunteer Membership costs just £2 per month Saving the small things that run the planet Follow us Search Sign in Register for free My basket (0) Contact Home About Bugs What We Do Get Involved News & Blog Advice Shop You are here: Home > About Bugs > Water measurer See more bugs Water measurer Select species Fast facts Buglife Latin name: Hydrometra stagnorum e-newsletter Notable feature: These animals have hydrophobic Keep up to date on (water fearing) hairs on campaigns, events and their undersides or on news with the Buglife their legs e-newsletter Rarity in UK: Rare / Common Sign up here Common water-measurer (Hydrometra stagnorum) Oil beetle hunt © (c) Entomart Your sightings Water-measurers are so called because they can be Log in to submit your watched slowly walking around on the surface of Seefindings the map ditches and ponds, apparently pacing out the distances between points! Water-measurers are part of the insect Bug identifier ‘boat’ community - whirligig beetles, water crickets, pond skaters and water-measurers all live on top of https://www.buglife.org.uk/bugs-and-habitats/water-measurer 24/02/2016 Water measurer | Buglife Page 2 of 5 What's that the water surface. All these animals have hydrophobic bug? (water fearing) hairs on their undersides or on their Use our Q&A legs. The repulsive force between the water and these to identify UK hair are sufficient to support the weight of the insects Identifybugs. bugs on the water surface. Bug facts 60% of all invertebrate species are declining More bug facts For younger bug lovers Download Bug Buddies Packed with fun activities, guides and Downloadbug info (PDF) https://www.buglife.org.uk/bugs-and-habitats/water-measurer 24/02/2016 Water measurer | Buglife Page 3 of 5 These insects are scavengers or carnivores which feed on bodies of small animals which land on or rise up to the water’s surface - dead or alive. -

Crossness Sewage Treatment Works Nature Reserve & Southern Marsh Aquatic Invertebrate Survey
Commissioned by Thames Water Utilities Limited Clearwater Court Vastern Road Reading RG1 8DB CROSSNESS SEWAGE TREATMENT WORKS NATURE RESERVE & SOUTHERN MARSH AQUATIC INVERTEBRATE SURVEY Report number: CPA18054 JULY 2019 Prepared by Colin Plant Associates (UK) Consultant Entomologists 30a Alexandra Rd London N8 0PP 1 1 INTRODUCTION, BACKGROUND AND METHODOLOGY 1.1 Introduction and background 1.1.1 On 30th May 2018 Colin Plant Associates (UK) were commissioned by Biodiversity Team Manager, Karen Sutton on behalf of Thames Water Utilities Ltd. to undertake aquatic invertebrate sampling at Crossness Sewage Treatment Works on Erith Marshes, Kent. This survey was to mirror the locations and methodology of a previous survey undertaken during autumn 2016 and spring 2017. Colin Plant Associates also undertook the aquatic invertebrate sampling of this previous survey. 1.1.2 The 2016-17 aquatic survey was commissioned with the primary objective of establishing a baseline aquatic invertebrate species inventory and to determine the quality of the aquatic habitats present across both the Nature Reserve and Southern Marsh areas of the Crossness Sewage Treatment Works. The surveyors were asked to sample at twenty-four, pre-selected sample station locations, twelve in each area. Aquatic Coleoptera and Heteroptera (beetles and true bugs) were selected as target groups. A report of the previous survey was submitted in Sept 2017 (Plant 2017). 1.1.3 During December 2017 a large-scale pollution event took place and untreated sewage escaped into a section of the Crossness Nature Reserve. The primary point of egress was Nature Reserve Sample Station 1 (NR1) though because of the connectivity of much of the waterbody network on the marsh other areas were affected. -

Heteroptera: Gerromorpha) in Central Europe
Shortened web version University of South Bohemia in České Budějovice Faculty of Science Ecology of Veliidae and Mesoveliidae (Heteroptera: Gerromorpha) in Central Europe RNDr. Tomáš Ditrich Ph.D. Thesis Supervisor: Prof. RNDr. Miroslav Papáček, CSc. University of South Bohemia, Faculty of Education České Budějovice 2010 Shortened web version Ditrich, T., 2010: Ecology of Veliidae and Mesoveliidae (Heteroptera: Gerromorpha) in Central Europe. Ph.D. Thesis, in English. – 85 p., Faculty of Science, University of South Bohemia, České Budějovice, Czech Republic. Annotation Ecology of Veliidae and Mesoveliidae (Hemiptera: Heteroptera: Gerromorpha) was studied in selected European species. The research of these non-gerrid semiaquatic bugs was especially focused on voltinism, overwintering with physiological consequences and wing polymorphism with dispersal pattern. Hypotheses based on data from field surveys were tested by laboratory, mesocosm and field experiments. New data on life history traits and their ecophysiological consequences are discussed in seven original research papers (four papers published in peer-reviewed journals, one paper accepted to publication, one submitted paper and one communication in a conference proceedings), creating core of this thesis. Keywords Insects, semiaquatic bugs, life history, overwintering, voltinism, dispersion, wing polymorphism. Financial support This thesis was mainly supported by grant of The Ministry of Education, Youth and Sports of the Czech Republic No. MSM 6007665801, partially by grant of the Grant Agency of the University of South Bohemia No. GAJU 6/2007/P-PřF, by The Research Council of Norway: The YGGDRASIL mobility program No. 195759/V11 and by Czech Science Foundation grant No. 206/07/0269. Shortened web version Declaration I hereby declare that I worked out this Ph.D. -

Anisus Vorticulus (Troschel 1834) (Gastropoda: Planorbidae) in Northeast Germany
JOURNAL OF CONCHOLOGY (2013), VOL.41, NO.3 389 SOME ECOLOGICAL PECULIARITIES OF ANISUS VORTICULUS (TROSCHEL 1834) (GASTROPODA: PLANORBIDAE) IN NORTHEAST GERMANY MICHAEL L. ZETTLER Leibniz Institute for Baltic Sea Research Warnemünde, Seestr. 15, D-18119 Rostock, Germany Abstract During the EU Habitats Directive monitoring between 2008 and 2010 the ecological requirements of the gastropod species Anisus vorticulus (Troschel 1834) were investigated in 24 different waterbodies of northeast Germany. 117 sampling units were analyzed quantitatively. 45 of these units contained living individuals of the target species in abundances between 4 and 616 individuals m-2. More than 25.300 living individuals of accompanying freshwater mollusc species and about 9.400 empty shells were counted and determined to the species level. Altogether 47 species were identified. The benefit of enhanced knowledge on the ecological requirements was gained due to the wide range and high number of sampled habitats with both obviously convenient and inconvenient living conditions for A. vorticulus. In northeast Germany the amphibian zones of sheltered mesotrophic lake shores, swampy (lime) fens and peat holes which are sun exposed and have populations of any Chara species belong to the optimal, continuously and densely colonized biotopes. The cluster analysis emphasized that A. vorticulus was associated with a typical species composition, which can be named as “Anisus-vorticulus-community”. In compliance with that both the frequency of combined occurrence of species and their similarity in relative abundance are important. The following species belong to the “Anisus-vorticulus-community” in northeast Germany: Pisidium obtusale, Pisidium milium, Pisidium pseudosphaerium, Bithynia leachii, Stagnicola palustris, Valvata cristata, Bathyomphalus contortus, Bithynia tentaculata, Anisus vortex, Hippeutis complanatus, Gyraulus crista, Physa fontinalis, Segmentina nitida and Anisus vorticulus. -

Zierliche Tellerschnecke (Anisus Vorticulus)
HESSEN-FORST Artensteckbrief Zierliche Tellerschnecke (Anisus vorticulus) Stand: 2006 weitere Informationen erhalten Sie bei: Hessen-Forst FENA Naturschutz Europastraße 10 - 12 35394 Gießen Tel.: 0641 / 4991-264 E-Mail: [email protected] Art Deutscher Name: Zierliche Tellerschnecke, Anisus (Disculifer ) vorticulus (T ROSCHEL 1834) Synonyme: Planorbis vorticulus, Spiralina vorticulus, Planorbis acies, Gyrorbis vorticulus, Planorbis charteus, Planorbis bavarica, Gyrorbis helveticus Systematische Einordnung Reich: Mollusca CUVIER 1795 Klasse: Gastropoda CUVIER 1795 Unterklasse: Orthogastropoda PELSENEER 1889 Überordnung: Heterobranchia J. E. GRAY 1840 Ordnung: Pulmonata CUVIER in BLAINVILLE 1814 Unterordnung: Basommatophora KEFERSTEIN 1864 Überfamilie: Planorboidea RAFINISQUE 1815 Familie: Planorbidae RAFINISQUE 1815 Unterfamilie: Planorbinae RAFINISQUE 1815 Gattung: Anisus S. STUDER 1820 Untergattung: Disculifer C. BOETTGER 1944 Verbreitung und Bestandsentwicklung Gesamt-Verbreitung: Die Gesamtart besiedelt Ost- und Mittel-Europa, die Britischen Inseln nur in Teilen (Sussex, Norfolk). Sie reicht im Süden bis ins Burgenland, nach Nord-Tirol, Vorarlberg und die Schweiz (Glöer 2002), im Westen mit nur wenigen verstreute Fundorten in Frankreich (Thonon, Rhone-Becken, Ried) (FALKNER & al. 2002), keine in Belgien, zahlreiche in den Niederlanden, vereinzelte in Süd-Dänemark (GLÖER 2002). Regionale Verbreitung: In Hessen ist Anisus vorticulus von einem einzigen Fundort bei Trebur (Hessisches Ried) bekannt (PETRY 1925). Dieses Vorkommen -

Observations on the Ecology of Water Striders
* RILEY f : Observations on the Ecology of Water Stridors Zoology M. S. 1912 MS/TV. <>V THE UNIVERSITY OF ILLINOIS LIBRARY ^5 OBSERVATIONS ON THE ECOLOGY OF WATER STRIDERS BY CHARLES FREDERICK CURTIS RILEY A. B. Doane College, 1901 A. B. University of Michigan, 1905 THESIS Submitted in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE IN ZOOLOGY IN THE GRADUATE SCHOOL OF THE UNIVERSITY OF ILLINOIS 1912 TH5 \vi_ r-4S UNIVERSITY OF ILLINOIS THE GRADUATE SCHOOL 19JT2J 1 HEREBY RECOMMEND THAT THE THESIS PREPARED UNDER MY SUPERVISION BY C: 31 C OIL* . ENTITLED IaJoJjjx- ^tX^MlA^ BE ACCEPTED AS FULFILLING THIS PART OF THE REQUIREMENTS FOR THE DEGREE OF In Charge of Major Work Recommendation concurred in: Committee on Final Examination Digitized by the Internet Archive in 2014 http://archive.org/details/observationsonecOOrile Observations on the Ecology of Water Stridors. Table of Contents. I. Introduction. II. Running Water Habitats 1. Ravines. 2. Springs. 5. Small Temporary Streams. a. Tile-drains and Temporary Streams. 4. Permanent Brooks. a. Behavior During Flood Conditions. b. Drought Conditions. c. Behavior During Conditions of Severe Drought. 5. Creeks. a. Dralnage-di tc h. 6. Rivers. a. Ox-bow Ponds. II. Food and Food Relations. I. Nature and Source of Focd. Food During Captivity. Transportation of Food by Water Currents. Time of Feeding. Response to Water Currents and its Relation to Food. Trial Method of Response to Moving Objects and Its Relation to Food. IV. Summary. V. Bibliography. -3- OESERVATIONS ON THE ECOLOGY OF WATER STRIDERS. I. Introduction. The present paper treats of some of the general ecological relations of water striders. -

Environmental Impact Report
ENVIRONMENTAL IMPACT REPORT SUPPLEMENT TO THE REPORT ON THE ENVIROMENTAL IMPACT OF THE “CONSTRUCTION OF THE KARCINO-SARBIA WIND FARM (17 WIND TURBINES)” OF 2003 Name of the undertaking: KARCINO-SARBIA Wind Farm (under construction) Contractor: AOS Agencja Ochrony Środowiska Sp. z o.o. based in Koszalin Arch. No. 52/OŚ/OOS/06 Koszalin, September 2006 Team: Bogdan Gutkowski, M.Sc.Eng.– Expert for Environmental Impact Assessment Appointed by the Governor of the West Pomerania Province Marek Ziółkowski, M.Sc. Eng. – Environmental Protection Expert of the Ministry of Environmental Protection, Natural Resources and Forestry; Environmental Protection Consultant Dagmara Czajkowska, M.Sc. Eng. – Specialist for Environmental Impact Assessment, Specialist for Environmental Protection and Management Ewa Reszka, M.Sc. – Specialist for the Protection of Water and Land and Protection against Impact of Waste Damian Kołek, M.Sc.Eng. – Environmental Protection Specialist 2 CONTENTS I. INTRODUCTION .................................................................................................................. 5 II. GENERAL INFORMATION ABOUT THE PROJECT ..................................................... 9 1. Location and adjacent facilities....................................................................................................... 9 2. Modifications to the project .......................................................................................................... 10 3. Technical description of the project .............................................................................................. -

Seznam Vodních Druhů Ploštic
SEZNAM VODNÍCH DRUH Ů PLOŠTIC (HETEROPTERA: NEPOMORPHA, GERROMORPHA) ČR PETR KMENT NEPOMORPHA Popov, 1968 NEPOIDEA Latreille, 1802 NEPIDAE Latreille, 1802 Nepinae Latreille, 1802 Nepini Latreille, 1802 Nepa cinerea Linnaeus, 1758 BM Ranatrinae Douglas & Scott, 1865 Ranatrini Douglas & Scott, 1865 Ranatra (Ranatra) linearis (Linnaeus, 1758) BM CORIXOIDEA Leach, 1815 CORIXIDAE Leach, 1815 Micronectinae Jaczewski, 1924 [Micronecta (Dichaetonecta) pusilla (Horváth, 1895)] Micronceta (Dichaetonecta) scholtzi (Fieber, 1860) BM Micronecta (Micronecta) griseola Horváth, 1899 BM Micronecta (Micronecta) minutissima (Linnaeus, 1758) BM Micronecta (Micronecta) poweri poweri (Douglas & Scott, 1869) BM Cymatiainae Walton, 1940 Cymatia bonsdorffii (C. R. Sahlberg, 1819) BM Cymatia coleoptrata (Fabricius, 1777) BM Cymatia rogenhoferi (Fieber, 1864) BM Corixinae Leach, 1815 Glaenocorisini Hungerford, 1948 Glaenocorisa propinqua (Fieber, 1860) BM Corixini Leach, 1815 Arctocorisa carinata carinata (C. R. Sahlberg, 1819) B Arctocorisa germari (Fieber, 1848) B Callicorixa praeusta praeusta (Fieber, 1848) BM Corixa affinis Leach, 1817 M Corixa dentipes Thomson, 1869 BM Corixa punctata (Illiger, 1807) BM Hesperocorixa castanea (Thomson, 1869) B Hesperocorixa linnaei (Fieber, 1848) BM Hesperocorixa moesta (Fieber, 1848) B Hesperocorixa sahlbergi (Fieber, 1848) BM Paracorixa concinna concinna (Fieber, 1848) BM Sigara (Halicorixa) stagnalis stagnalis (Leach, 1817) ?B Sigara (Microsigara) hellensii (C. R. Sahlberg, 1819) BM Sigara (Pseudovermicorixa) nigrolineata -

XVII. KONFERENCE České Limnologické Společnosti a Slovenskej Limnologickej Spoločnosti „VODA – VĚC VEŘEJNÁ“
XVII. KONFERENCE České limnologické společnosti a Slovenskej limnologickej spoločnosti „VODA – VĚC VEŘEJNÁ“ SBORNÍK PŘÍSPĚVKŮ 29. června – 3. července 2015, Mikulov Vanda Rádková a Jindřiška Bojková (eds.) Citace RÁDKOVÁ Vanda (ed.) a BOJKOVÁ Jindřiška (ed.). XVII. konference České limnologické společnosti a Slovenskej limnologickej spoločnosti „Voda – věc veřejná“: Sborník příspěvků. Brno: Masarykova univerzita, 2015. ISBN 978-80-210-7874-1. Všechna práva vyhrazena. Žádná část této elektronické knihy nesmí být reprodukována nebo šířena v papírové, elektronické či jiné podobě bez předchozího písemného souhlasu vykonavatele majetkových práv k dílu, kterého je možno kontaktovat na adrese – Nakladatelství Masarykovy univerzity, Žerotínovo náměstí 9, 601 77 Brno. Vanda Rádková a Jindřiška Bojková (eds.) XVII. konference České limnologické společnosti a Slovenskej limnologickej spoločnosti „Voda – věc veřejná“ Sborník příspěvků Vydala Masarykova univerzita, Žerotínovo nám. 617/9, 601 77 Brno 1. vydání, 2015 Tisk: GNT s.r.o., Purkyňova 1678/8, 612 00 Brno ISBN 978-80-210-7874-1 (brož. vaz.) ISBN 978-80-210-7990-8 (online : pdf) ÚVODNÍ SLOVO Milé kolegyně, kolegové, účastníci 17. konference ČLS a SLS, tři roky od poslední, společné konference našich limnologických společností uběhly doslova jako voda a nadchází proto čas se opět sejít a potkat s přáteli, jakož i novými tvářemi nastupující limnologické generace. Někdy se zdá, že tři roky je dlouhá doba. Častokrát jsme s kolegy ve výboru na toto téma diskutovali: měli bychom pořádat konference ve dvouletém intervalu nebo tříletý je dostatečný? Myslíme si, že na častější akce je nás málo, resp. jaksi nezbývá času. Takže tradice „jednou za tři roky“ bude pokračovat dál, i když s některými z vás se, bohužel, opravdu střetávám pouze během limnokonferencí. -

Characterisation of Pristine Polish River Systems and Their Use As Reference Conditions for Dutch River Systems
1830 Rapport 1367.qxp 20-9-2006 16:49 Pagina 1 Characterisation of pristine Polish river systems and their use as reference conditions for Dutch river systems Rebi Nijboer Piet Verdonschot Andrzej Piechocki Grzegorz Tonczyk´ Malgorzata Klukowska Alterra-rapport 1367, ISSN 1566-7197 Characterisation of pristine Polish river systems and their use as reference conditions for Dutch river systems Commissioned by the Dutch Ministry of Agriculture, Nature and Food Quality, Research Programme ‘Aquatic Ecology and Fisheries’ (no. 324) and by the project Euro-limpacs which is funded by the European Union under2 Thematic Sub-Priority 1.1.6.3. Alterra-rapport 1367 Characterisation of pristine Polish river systems and their use as reference conditions for Dutch river systems Rebi Nijboer Piet Verdonschot Andrzej Piechocki Grzegorz Tończyk Małgorzata Klukowska Alterra-rapport 1367 Alterra, Wageningen 2006 ABSTRACT Nijboer, Rebi, Piet Verdonschot, Andrzej Piechocki, Grzegorz Tończyk & Małgorzata Klukowska, 2006. Characterisation of pristine Polish river systems and their use as reference conditions for Dutch river systems. Wageningen, Alterra, Alterra-rapport 1367. 221 blz.; 13 figs.; 53 tables.; 26 refs. A central feature of the European Water Framework Directive are the reference conditions. The ecological quality status is determined by calculating the distance between the present situation and the reference conditions. To describe reference conditions the natural variation of biota in pristine water bodies should be measured. Because pristine water bodies are not present in the Netherlands anymore, water bodies (springs, streams, rivers and oxbow lakes) in central Poland were investigated. Macrophytes and macroinvertebrates were sampled and environmental variables were measured. The water bodies appeared to have a high biodiversity and a good ecological quality. -
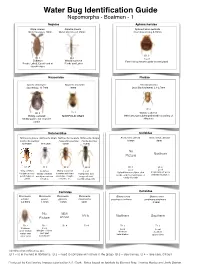
Water Bug ID Guide
Water Bug Identification Guide Nepomorpha - Boatmen - 1 Nepidae Aphelocheridae Nepa cinerea Ranatra linearis Aphelocheirus aestivalis Water Scorpion, 20mm Water Stick Insect, 35mm River Saucer bug, 8-10mm ID 1 ID 1 ID 1 Local Common Widely scattered Fast flowing streams under stones/gravel Ponds, Lakes, Canals and at Ponds and Lakes stream edges Naucoridae Pleidae Ilycoris cimicoides Naucoris maculata Plea minutissima Saucer bug, 13.5mm 10mm Least Backswimmer, 2.1-2.7mm ID 1 ID 1 ID 2 Widely scattered Widely scattered NORFOLK ONLY Often amongst submerged weed in a variety of Muddy ponds and stagnant stillwaters canals Notonectidae Corixidae Notonecta glauca Notonecta viridis Notonecta maculata Notonecta obliqua Arctocorisa gemari Arctocorisa carinata Common Backswimmer Peppered Backswimmer Pied Backswimmer 8.8mm 9mm 14-16mm 13-15mm 15mm 15mm No Northern Picture ID 2 ID 2 ID 2 ID 2 ID 3 ID 3 Local Local Very common Common Widely scattered Local Upland limestone lakes, dew In upland peat pools Ubiquitous in all Variety of waters In waters with hard Peat ponds, acid ponds, acid moorland lakes, or with little vegetation ponds, lakes or usually more base substrates, troughs, bog pools and sandy silt ponds canals rich sites concrete, etc. recently clay ponds Corixidae Corixidae Micronecta Micronecta Micronecta Micronecta Glaenocorisa Glaenocorisa scholtzi poweri griseola minutissima propinqua cavifrons propinqua propinqua 2-2.5mm 1.8mm 1.8mm 2mm 8.3mm No NEW RARE Northern Southern Picture ARIVAL ID 2 ID 2 ID 4 ID 4 ID 3 ID 3 Local Common Local Local Margins of rivers open shallow Northern Southern and quiet waters over upland lakes upland lakes silt or sand backwaters Identification difficulties are: ID 1 = id in the field in Northants.