Ex-Vivo Slaughterhouse Porcine Crystalloid-Perfused Beating Heart Via Langendorff Method
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

S41467-021-21178-4.Pdf
ARTICLE https://doi.org/10.1038/s41467-021-21178-4 OPEN The mechanosensitive Piezo1 channel mediates heart mechano-chemo transduction Fan Jiang1, Kunlun Yin2, Kun Wu 1,5, Mingmin Zhang1, Shiqiang Wang3, Heping Cheng 4, Zhou Zhou2 & ✉ Bailong Xiao 1 The beating heart possesses the intrinsic ability to adapt cardiac output to changes in mechanical load. The century-old Frank–Starling law and Anrep effect have documented that 1234567890():,; stretching the heart during diastolic filling increases its contractile force. However, the molecular mechanotransduction mechanism and its impact on cardiac health and disease remain elusive. Here we show that the mechanically activated Piezo1 channel converts mechanical stretch of cardiomyocytes into Ca2+ and reactive oxygen species (ROS) sig- naling, which critically determines the mechanical activity of the heart. Either cardiac-specific knockout or overexpression of Piezo1 in mice results in defective Ca2+ and ROS signaling and the development of cardiomyopathy, demonstrating a homeostatic role of Piezo1. Piezo1 is pathologically upregulated in both mouse and human diseased hearts via an autonomic response of cardiomyocytes. Thus, Piezo1 serves as a key cardiac mechanotransducer for initiating mechano-chemo transduction and consequently maintaining normal heart function, and might represent a novel therapeutic target for treating human heart diseases. 1 State Key Laboratory of Membrane Biology, Tsinghua-Peking Center for Life Sciences, Beijing Advanced Innovation Center for Structural Biology, IDG/ McGovern Institute for Brain Research, School of Pharmaceutical Sciences, Tsinghua University, Beijing 100084, China. 2 State Key Laboratory of Cardiovascular Disease, Beijing Key Laboratory for Molecular Diagnostics of Cardiovascular Diseases, Center of Laboratory Medicine, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100037, China. -

Mechanical Model of the Left Ventricle of the Heart Approximated by Axisymmetric Geometry
pp. 1–14 (2017) Mechanical model of the left ventricle of the heart approximated by axisymmetric geometry F. A. Syomin∗ and A. K. Tsaturyan∗ Abstract — An axisymmetric model is suggested to simulate mechanical performance of the left vent- ricle of the heart. Cardiac muscle is treated as incompressible anisotropic material with active tension directed along muscle fibres. This tension depends on kinetic variables that characterize interaction of contractile proteins and regulation of muscle contraction by calcium ions. For numerical simulation of heartbeats the finite element method was implemented. The model reproduces well changes in vent- ricle geometry between systole and diastole, ejection fraction, pulse wave of ventricular and arterial pressure typical for normal human heart. The model also reproduces well the dependence of the stroke volume on end-diastolic and arterial pressures (the Frank–Starling law of the heart and Anrep effect). The results demonstrate that our model of cardiac muscle can be successfully applied to multiscale 3D simulation of the heart. Keywords: Heart, cardiac muscle, muscle contraction, mathematical model, finite elements. MSC 2010: 74L15, 92C10, 92C30, 74S05 Computer modelling of the heart mechanics is a fast developing field of computa- tional physiology. During the last two decades a number of electromechanical mod- els of the whole heart or its left ventricle has been developed [26]. Such multiscale models usually combine several models that describe electrical and chemical pro- cesses at the level of a single cell with mechanical properties of cardiac muscle tissue. Generally, a model of the heart consists of a model of ionic currents in the cardiomyocytes, model of myocardial mechanics, model of blood circulation (hae- modynamics model), and a geometrical approximation of the heart. -
15-Coned-Ccrn-Cardiovascular.Pdf
1 Cardiovascular Introduction A. Anatomy & Physiology B. Cardiac Assessment 1. Cardiac Risk Factors Non Modifiable Age Gender Family History Race Modifiable Smoking Hypertension Diabetes Obesity Stress Exercise Hyperlipidemia 2. Medical & Surgical History 3. Social History 4. Medication History 5. Physical Exam 2 Color Pulses Rate & Rhythm PMI Location Extremity Temperature Dyspnea Fatigue Level Fluid Retention Palpitations Dizziness 6. Chest Pain Exam PQRST Assessment P: Pain, Placement, Provocation Q: Quality (sharp, stabbing, pressure) , Quantity R: Radiation, Relief S: Severity, Systems (nausea, sweaty, dizziness) T: Timing (when it started, how long did it last, what makes it better or worse) C. Diagnostic Tests & Procedures 1. 12 Lead ECG 2. Echocardiography (Transthoracic and Transesepheal) 3. Stress Test 4. Cardiac Catheterization 5. Doppler Ultrasound 6. Blood Work Acute Coronary Syndrome Cardiac Enzymes: CK-MB, Amino Acids: Troponins Heme Proteins: Myoglobin Lipid Profile Triglycerides Cholesterol Low Density Lipoproteins High Density Lipoprotein Coagulation Profile PT/INR aPTT ACT 3 Misc B Type Naturetic Peptide – BNP C Reactive Protein Homocysteine Hemodynamic Monitoring/Assessment CARDIAC OUTPUT HEART RATE X STROKE VOLUME PRELOAD + AFTERLOAD + CONTRACTILITY + The volume of blood in the The pressure or resistance the LV must contract The ability of the against or overcome to eject the blood or create ventricle at end diastole. systole. myocardium to contract. Total Blood Volume -

Heart Failure Therapy: Potential Lessons Heart: First Published As 10.1136/Heartjnl-2015-309110 on 23 December 2016
Heart Online First, published on December 23, 2016 as 10.1136/heartjnl-2015-309110 Review ‘End-stage’ heart failure therapy: potential lessons Heart: first published as 10.1136/heartjnl-2015-309110 on 23 December 2016. Downloaded from from congenital heart disease: from pulmonary artery banding and interatrial communication to parallel circulation Dietmar Schranz, Hakan Akintuerk, Norbert F Voelkel ▸ Additional material is ABSTRACT reflected by the calculated transpulmonary gradient published online only. To view The final therapy of ‘end-stage heart failure’ is (TPG=mean PAP−LAP) and in particular the dia- please visit the journal online (http://dx.doi.org/10.1136/ orthotopic heart, lung or heart-lung transplantation. stolic PAP to LAP difference, the so-called diastolic heartjnl-2015-309110). However, these options are not available for many pressure gradient (DPG=diastolic PAP−LAP). Both patients worldwide. Therefore, novel therapeutical parameters become even more important in the Pediatric Heart Center, Justus Liebig University Giessen, strategies are needed. Based on pathophysiological setting of heart failure: pulmonary hypertension Virginia Commonwealth insights regarding (1) the long-term impact of an (PH) associated with an increased LAP, but normal University, School of Pharmacy, obstructive pulmonary outflow tract in neonates with TPG (<12 mm Hg) and in particular DPG Richmond, Virginia, USA congenitally corrected transposition of the great arteries, (<7 mm Hg)—labelled isolated postcapillary pul- — Correspondence to (2) the importance of a restrictive versus a non-restrictive monary hypertension may increase RV afterload Professor Dietmar Schranz, atrial septum in neonates born with a borderline left and negatively affect right (subpulmonary) ven- Pediatric Heart Center, Justus ventricle and (3) the significance of both, a patent tricular function. -

Core Topics in Cardiac Anesthesia, Second Edition
Cambridge University Press 978-0-521-19685-7 - Core Topics in Cardiac Anesthesia: Second Edition Edited by Jonathan H. Mackay and Joseph E. Arrowsmith Index More information Index abciximab 465–6 airway management, postoperative endocarditis therapy 449 abdominal arteries 79 resuscitation 416 multi-resistant bacteria 450–2 accessory conducting pathways aldosterone 31–2 surgical prophylaxis 246, 342, 362, 9–10 alfentanil 36–7 447–8 acetylcholine (ACh) 25 allograft vasculopathy 280 anticoagulation acid-base management 391, 403–4, allostasis 29 atrial fibrillation 412 441 alpha-1-microalbumin 435 CPB 64–7, 175 actin 13–15 alpha-adrenergic receptors (a-ARs) ECMO 364 action potentials (APs), cardiac 8–11 25, 41, 48 endogenous 367–8 activated clotting time (ACT) 64, 67, alpha-stat blood-gas management 391, intra-aortic balloon pump 357 175, 370 403–4, 441 monitoring 67, 175 activated partial thromboplastin time e-aminocaproic acid (EACA) 68, 69, off-pump coronary surgery (APPT) 67 175, 368 190–1 acute coronary syndromes (ACS) amiodarone 57, 60 preoperative cessation 169 257–60 amitriptyline 463 prosthetic valve recipients 127, anesthetic management 258 Amplatzer muscular VSD 129–30 pathogenesis 257 occluder 264 antidepressants 463 acute kidney injury (AKI) 431–6 Amplatzer septal occluder 262–3 antidiuretic hormone (ADH) 31–2 acute lung injury (ALI) 421 analgesia anti-dysrhythmic drugs 57–62, 256, acute normovolemic hemodilution chronic pain 463 409 (ANH) 372, 468 postoperative 182, 191, 238–9, antifibrinolytics 68–9, 368–9 acute renal failure -

The Role of Heart Rate on Functional Capacity In
The role of heart rate on functional capacity in chronic heart failure: association or contribution? Haqeel Ahmed Jamil Submitted in accordance with the requirements for the degree of Doctor of Philosophy The University of Leeds Leeds Institute for Cardiovascular and Metabolic Medicine School of Medicine September 2015 - ii - Intellectual Property and Publication Statements: The candidate confirms that the work submitted is his own and that appropriate credit has been given where reference has been made to the work of others. This copy has been supplied on the understanding that it is copyright material and that no quotation from the thesis may be published without proper acknowledgement. The right of Haqeel Ahmed Jamil to be identified as Author of this work has been asserted by him in accordance with the Copyright, Designs and Patents Act 1988. © 2015 The University of Leeds and Haqeel Ahmed Jamil - iii - Dedication This thesis is affectionately dedicated to my parents, Mohammed and Azra Jamil for their unremitting love and wisdom, and without whom none of my career would have been possible; to my brothers, Adeel and Nabeel for always providing a mixture of encouragement and welcome distractions; to my wife Sana for her selfless support and understanding throughout: and to my son Zackaria, for the joy he brings to all of our lives. - iv - Acknowledgements I would like to start by thanking my supervisors, Klaus Witte and Mark Kearney for recognising my potential, constantly inspiring me and allowing me the opportunity to undertake research at the Leeds Institute for Cardiovascular and Metabolic Medicine (LICAMM). I would also like to sincerely thank the LICAMM research team and all the staff at the Leeds Cardiac Investigations Unit, for their assistance and support. -
Keys to Successful Cardioversion
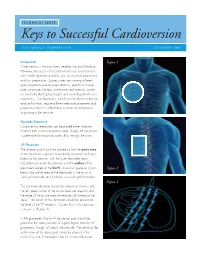
TECHNICAL NOTE: Keys to Successful Cardioversion ZOLL MEDICAL CORPORATION DECEMBER 2009 Introduction Figure 1 Cardioversion is the most direct treatment for atrial fibrillation. However, the success of a cardioversion can be enhanced with careful attention to details, such as electrode placement and skin preparation. Success rates vary among different types of patients and among institutions, and this technical note summarizes the keys to enhance cardioversion success as shared by electrophysiologists and cardiologists with vast experience. Cardioversions, which are intended to terminate atrial arrhythmias, require different electrode placement and preparation than for defibrillation to terminate arrhythmias originating in the ventricle. Electrode Placement Cardioversion electrodes can be placed either Anterior– Posterior (AP) or Anterior-Anterior (AA), though AP placement is preferable for maximum current flow through the atria. AP Placement The anterior pad should be placed so that the active area of the electrode is placed immediately adjacent and right lateral to the sternum, with the outer electrode foam circumference under the clavicle and the midline of the electrode located at the fourth intracostal space as shown Figure 2 below (the active area of the electrodes is the tin on a solid gel electrode and the foam on a wet gel electrode). Figure 3 The posterior electrode should be placed as shown, with the left upper corner of the active area sub-scapular and the edge of the active area immediately left lateral to the spine. The center of the electrode should be placed at the level of the T7 vertebra. Current flow in this position is shown in (Figure 3) . In AA placement (Figure 4) the sternal pad should be placed in the same position or slightly higher than for AP placement, though still below the clavicle. -

State of Missouri Physician Manual
STATE OF MISSOURI PHYSICIAN MANUAL Physician SECTION 1-PARTICIPANT CONDITIONS OF PARTICIPATION ........................................22 1.1 INDIVIDUALS ELIGIBLE FOR MO HEALTHNET, MANAGED CARE OR STATE FUNDED BENEFITS........................................................................................................................22 1.1.A DESCRIPTION OF ELIGIBILITY CATEGORIES.............................................................22 1.1.A(1) MO HealthNet...............................................................................................................22 1.1.A(2) MO HealthNet for Kids.................................................................................................23 1.1.A(3) Temporary MO HealthNet During Pregnancy (TEMP)................................................25 1.1.A(4) Voluntary Placement Agreement for Children .............................................................25 1.1.A(5) State Funded MO HealthNet.........................................................................................25 1.1.A(6) MO Rx...........................................................................................................................26 1.1.A(7) Women’s Health Services .............................................................................................26 1.1.A(8) ME Codes Not in Use ...................................................................................................27 1.2 MO HEALTHNET AND MO HEALTHNET MANAGED CARE ID CARD......................27 1.2.A FORMAT OF MO HEALTHNET -

AACN Essentials of Critical-Care Nursing Pocket Handbook, Second
AACN Essentials of Critical Care Nursing—Pocket Handbook Notice Medicine is an ever-changing science. As new research and clinical experience broaden our knowledge, changes in treatment and drug therapy are required. The editor and publisher of this work have checked with sources believed to be reliable in their efforts to provide information that is complete and generally in accord with the standards accepted at the time of publication. However, in view of the possibility of human error or changes in medical sciences, neither the editors nor the publisher nor any other party who has been involved in the preparation or publication of this work warrants that the information contained herein is in every respect accurate or complete, and they disclaim all responsibility for any errors or omissions or for the results obtained from use of the information contained in this work. Readers are encouraged to confirm the information contained herein with other sources. For example and in particular, readers are advised to check the product information sheet included in the package of each drug they plan to administer to be certain that the information contained in this work is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. This recommendation is of particular importance in connection with new or infrequently used drugs. AACN Essentials of Critical Care Nursing Pocket Handbook Second Edition Marianne Chulay, RN, PhD, FAAN Consultant, Critical Care Nursing and Clinical Research Gainesville, Florida Suzanne M. Burns RN, MSN, RRT, ACNP, CCRN, FAAN, FCCM, FAANP Professor of Nursing, Acute and Specialty Care School of Nursing Advanced Practice Nurse Level 2, Director Professional Nursing Staff Organization Research Program University of Virginia Health System Charlottesville, Virginia New York Chicago San Francisco Lisbon London Madrid Mexico City Milan New Delhi San Juan Seoul Singapore Sydney Toronto Copyright © 2010, 2006 by The McGraw-Hill Companies, Inc. -

Thrombospondin-4 Is Required for Stretch-Mediated Contractility Augmentation in Cardiac Muscle Oscar H
Thrombospondin-4 Is Required for Stretch-Mediated Contractility Augmentation in Cardiac Muscle Oscar H. Cingolani, Jonathan A. Kirk, Kinya Seo, Norimichi Koitabashi, Dong-ik Lee, Genaro Ramirez-Correa, Djahida Bedja, Andreas S. Barth, An L. Moens and David A. Kass Circ Res. 2011;109:1410-1414; originally published online October 27, 2011; doi: 10.1161/CIRCRESAHA.111.256743 Circulation Research is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231 Copyright © 2011 American Heart Association, Inc. All rights reserved. Print ISSN: 0009-7330. Online ISSN: 1524-4571 The online version of this article, along with updated information and services, is located on the World Wide Web at: http://circres.ahajournals.org/content/109/12/1410 Data Supplement (unedited) at: http://circres.ahajournals.org/content/suppl/2011/10/27/CIRCRESAHA.111.256743.DC1.html Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Circulation Research can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer document. Reprints: Information about reprints can be found online at: http://www.lww.com/reprints Subscriptions: Information about subscribing to Circulation Research is online at: http://circres.ahajournals.org//subscriptions/ Downloaded from http://circres.ahajournals.org/ at SWETS SUBSCRIPTION SERVICE on November 25, 2013 Integrative Physiology Thrombospondin-4 Is Required for Stretch-Mediated Contractility Augmentation in Cardiac Muscle Short Communication Oscar H. -
Mastering Pacing, Cardioversion and Defibrillation
Mastering Pacing, Cardioversion and Defibrillation Mike McEvoy, PhD, NRP, RN, CCRN Sr. Staff RN – Cardiovascular Surgical ICU Albany Medical Center NTI class code: EXED223 Pacing, Cardioversion, Defib Indications Too many to list… (Patient Must Be Symptomatic and Documented with ECG) Too slow Too fast Too irregular Unstable VT Cardiovert or Defibrillate? Cardioversion is synchronized with… R on T Leads? Pads? Do you need to place the leads? Where’s the best place for the pads? Synchronized Cardioversion Now, too slow… Pacing pads can be applied any time! Better to have them in place before you need them – easier to move the patient Transcutaneous Pacing Pads Conduct through skin Transcutaneous Set Rate Set Output TCP – Transcutaneous Pacing Pads on patient –Leads usually needed Turn on Pacing –Starts @ 0 mA, 80/min Increase mA until capture observed Confirm mechanical capture (pulse, SpO2) Capture TCP: What Mode? VOO VVI What’s the problem/solution? Loss of capture – increase mA Stimulation Threshold The minimum output needed to consistently capture the heart 100 mA 75 mA 50 mA Something’s not right here… Intrinsic Beat Paced Beat Intrinsic Beat Paced Beat Fusion Beat Pseudofusion Beat Fusion Beat Pseudofusion Beat Pause Button Drops rate to 25% programed value Won’t STOP pacing (10 ppm minimum) Really bad - vfib Rule # 1: Show Up & Shock Goal for defibrillation: Hospital: 3 minutes Community: 5 minutes Albert Einstein “The definition of insanity is doing the same thing over and over again and expecting different results.” Rule #2: Think Birthday Cake Optimal defib field = 90% myocardium What would Einstein do? energy Change pad location NTI class code: EXED223 Mike McEvoy [email protected] [email protected] www.mikemcevoy.com @mcevoymike mcevoymike. -

The Anrep Effect Reconsidered
The Anrep Effect Reconsidered R. G. Monroe, … , C. Phornphutkul, M. Davis J Clin Invest. 1972;51(10):2573-2583. https://doi.org/10.1172/JCI107074. Research Article Evidence is presented supporting the hypothesis that the positive inotropic effect after an abrupt increase in systolic pressure (Anrep effect) is the recovery from subendocardial ischemia induced by the increase and subsequently corrected by vascular autoregulation of the coronary bed. Major evidence consists of data obtained from an isolated heart preparation showing that the Anrep effect can be abolished with coronary vasodilation, and that with an abrupt increase in systolic pressure there is a significant reduction in the distribution of coronary flow to subendocardial layers of the ventricle. Furthermore, the intracardiac electrocardiogram shows S-T segment and T wave changes after an abrupt increase in ventricular pressure similar to that noted after coronary constriction. Major implications are that normally there may be ischemia of the subendocardial layers tending to reduce myocardial contractility which may account, in part, for the positive inotropic effect of various coronary vasodilators; that with an abrupt increase in ventricular pressure the subendocardium is rendered temporarily ischemic, placing the heart in jeopardy from arrhythmias until this is corrected; and that end-diastolic pressure and the intracardiac electrocardiogram may provide a means of evaluating the adequacy of circulation to subendocardial layers in diseased ventricles when systolic pressure is abruptly increased. Find the latest version: https://jci.me/107074/pdf rhe Anrep Effect Reconsidered R. G. MONROE, W. J. GAMBLE, C. G. LAFARGE, A. E. KUMAR, J. STARK, G. L. SANDERS, C.