Assessment of Canine Elbow Joint for Osteoarthritis and Treatment with Synovetin OA®
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
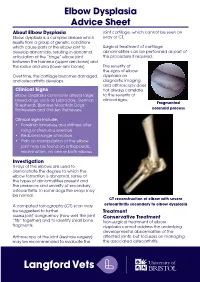
Elbow Dysplasia Advice Sheet About Elbow Dysplasia Joint Cartilage, Which Cannot Be Seen on Elbow Dysplasia Is a Complex Disease Which X-Ray Or CT
Elbow Dysplasia Advice Sheet About Elbow Dysplasia joint cartilage, which cannot be seen on Elbow dysplasia is a complex disease which x-ray or CT. results from a group of genetic conditions which cause parts of the elbow joint to Surgical treatment of cartilage develop abnormally, resulting in abnormal abnormalities can be performed as part of articulation of the “hinge” elbow joint this procedure if required. between the humerus (upper arm bone) and the radius and ulna (lower arm bones). The severity of the signs of elbow Over time, the cartilage becomes damaged, dysplasia on and osteoarthritis develops. diagnostic imaging and arthroscopy does Clinical Signs not always correlate Elbow dysplasia commonly affects large to the severity of breed dogs, such as Labradors, German clinical signs. Fragmented Shepherds, Bernese Mountain Dogs, Rottweilers and Golden Retrievers. coronoid process Clinical signs include; • Forelimb lameness and stiffness after rising or strenuous exercise • Reduced range of motion • Pain on manipulation of the elbow joint may be found on orthopaedic examination, on one or both elbows Investigation X-rays of the elbows are used to demonstrate the degree to which the elbow formation is abnormal, some of the types of abnormalities present and the presence and severity of secondary osteoarthritis. In some dogs the x-rays may be normal. CT reconstruction of elbow with severe A computed tomography (CT) scan may osteoarthritis secondary to elbow dysplasia be suggested to further Treatment assess joint congruency (how well the joint Conservative Treatment “fits” together) and to identify small bone Non-surgical treatment of elbow fragments. dysplasia cannot address the underlying developmental abnormalities of the Arthroscopy of the joint (keyhole surgery) affected joints, but focusses on managing may be recommended to evaluate the the associated osteoarthritis. -

Surgical Treatment of Canine Elbow Dysplasia (MCPD, UAP, OCD, EI)
PROCEEDINGS 33rd annual meeting of the INTERNATIONAL ELBOW WORKING GROUP September 24th 2018 WSAVA-FASAVA congress, Marina Bay Sands Congress Venue, Singapore. Welcome at the 33rd meeting of the International Elbow Working Group The International Elbow Working Group (IEWG) is an affiliate of the World Small Animal Veterinary Association (WSAVA). The board of the IEWG is therefore very grateful to the organisers of the 43rd WSAVA congress and the 9th FASAVA congress, in particular to Dr Shane Ryan, chairman of the Congress Committee, and Dr Frédéric Gaschen, chairman of the Congress Scientific Program Committee to be invited and facilitated in organizing a seminar during the pre-congress meeting of the WSAVA-2018 congress. By this invitation, the WSAVA underpins the importance of the role of the IEWG to perform the task of exchanging important information to the world veterinary community regarding the causes, prevalence, screening, therapy and prevention of one of the most important non-traumatic diseases of the locomotion system in dogs. The IEWG fulfils this task by organizing annual meetings with the help of esteemed scientist in the field of genetics, radiology, orthopedic surgery, and related fields to present the newest science and overviews on Elbow Dysplasia. Earlier this month, the IEWG had a seminar at the World Veterinary Orthopedic Congress, a joined meeting of the European Society of Veterinary Orthopedics and Traumatology (ESVOT) and the American Veterinary Orthopedic Society (VOS), with participants of European countries and the America’s. The IEWG is happy to be able to share new information with participants of the Asian and other countries coming together in Singapore. -

Sokoto Journal of Veterinary Sciences Preliminary Evaluation Of
Sokoto Journal of Veterinary Sciences, Volume 17 (Number 2). June, 2019 RESEARCH ARTICLE Sokoto Journal of Veterinary Sciences (P-ISSN 1595-093X: E-ISSN 2315-6201) http://dx.doi.org/10.4314/sokjvs.v17i2.6 Ajadi & Doyin-Dada./Sokoto Journal of Veterinary Sciences, 17(2): 45 - 53. Preliminary evaluation of prevalence of hip and elbow dysplasia in Boerboel dogs RA Ajadi *& OA Doyin-Dada Department of Veterinary Medicine and Surgery, Federal University of Agriculture, Abeokuta PMB 2240, Alabata Road, Abeokuta, Ogun State, Nigeria *Correspondence: Tel.: +2347033800326; E-mail: [email protected] Copyright: © 2019 Abstract Ajadi & Doyin-Dada. Hip dysplasia (HD) and elbow dysplasia (ED) are developmental diseases that affect This is an open-access large breed of dogs disproportionately. Despite the large size of Boerboel dogs, there article published under are no breed prevalence for HD and ED in Nigeria. This study provides preliminary the terms of the information about HD and ED prevalence in Boerboels. Twenty Boerboels of both Creative Commons sexes were evaluated. Ventrodorsal radiographs of the hip joint and flexed lateral Attribution License radiographs of elbow joint were made from each dog, using digital technology. Hip which permits grading was done using the Fédération Cynologique Internationale system (World unrestricted use, Canine Organization), assigning grades ranging from A - E. Elbow radiographs were distribution, and graded based on the International Elbow Working Group criteria, and scores ranging reproduction in any from 0-3 were assigned. Prevalence of HD and ED were expressed as percentages. medium, provided the original author and Age and sex difference were compared using a chi square test. -

Elbow Dysplasia in Dogs
Elbow dysplasia in dogs Revised by Gary Clayton Jones BVetMed DSAO DVR FRCVS with additional graphics by Jonathan Clayton Jones The British Veterinary Association and the Kennel Club — working together for excellence in canine health Elbow dysplasia has been identified as a significant problem in many breeds. Importantly, the condition appears to be increasing worldwide. It begins in puppyhood, and can affect the dog for the rest of its life The ‘flexed mediolateral’ view is a side-on view of the elbow. This view allows examination of the secondary changes in ED which occur in the shaded areas. Note how some of the shaded areas here are overlaid by other structures, which makes them difficult to examine eterinary surgeons have been Once the dog reaches skeletal maturity disease in that they have primary lesions aware for many years of a number the primary lesions may stabilise. However, or osteoarthritis in their elbows but do not of conditions that begin in puppies once abnormal development has started appear obviously lame. Some dogs will be and cause lameness. Hip dysplasia with a primary lesion, further secondary symmetrically lame in each foreleg, which Vwas the first such disease to be widely changes follow, in particular, abnormal wear can be very difficult to see. Fortunately, these recognised and a scheme for its assessment of the joint surfaces and osteoarthritis subclinical dogs can often be identified by and control is well established in the UK. (sometimes termed arthrosis, or degenerative taking radiographs (X-ray images) of their Elbow dysplasia (ED) is a significant problem joint disease — DJD). -

Proceedings 2012
PROCEEDINGS 27th annual meeting of the INTERNATIONAL ELBOW WORKING GROUP April 11th 2012 ICC, hall 7b Birmingham, UK The International Elbow Working Group acknowledges the financial support by HILL’S PET NUTRITION 27th annual meeting IEWG, Birmingham UK, April 11th 2012, p 2 WELCOME ADDRESS Birmingham, April 2012 Dear IEWG-congress participant, The International Elbow Working Group (IEWG) has been founded by a group of veterinarians and dog breeders in Davis, CA, U.S.A. in 1989 with the aim to increase the knowledge on and awareness of elbow disease in dogs, and to support all stakeholders in disseminating new knowledge in this field. The interest in hereditary aspects of elbow dysplasia (ED) has been increasing ever since both among breeders and veterinary surgeons and radiologists alike. ED has been recognized as a cause of lameness in a large variety of dog breeds with an incidence as high as 50% of the screened dogs. Published data on prevalence vary depending on breed and country. These discrepancies can be a matter of screening bias (e.g. not including dogs suffering from the disease and treated at a young age, or not submitting films of affected dogs for official screening), of improvement gained by the implementation of breeding restrictions for affected dogs, or may be caused by different screening or grading modes. The latter emphasizes the necessity of uniform grading, preferably by the protocol introduced and refined by IEWG and in use in many countries for many years. Some research groups are in a process of performing molecular genetic research in the field of canine elbow dysplasia, to use the results in the future for screening of potential breeding stock. -

Angular Limb Deformities in Foals: Treatment and Prognosis*
Article #4 CE Angular Limb Deformities in Foals: Treatment and Prognosis* Nicolai Jansson, DVM, PhD, DECVS Skara Equine Hospital Skara, Sweden Norm G. Ducharme, DVM, MSc, DACVS Cornell University ABSTRACT: This article presents an overview of the clinical management of foals with angular limb deformities. Both conservative and surgical treatment options exist; the choice of which to use should be based on the type, severity, and location of the deformity as well as the age of the foal. Conservative measures include controlled exercise, rigid external limb support, and corrective hoof trimming. Surgical treatment modalities comprise tech- niques for manipulating physeal growth and, after physeal closure, various corrective osteotomy or ostectomy methods. The prognosis is generally good if treatment is initi- ated well in advance of physeal closure. ngular limb deformities and their treat- Conservative Treatment ment in foals and young horses constitute In most foals born with mild to moderate a significant part of the orthopedic prob- angular deformities, spontaneous resolution A 2 lems that veterinarians must manage. This article occurs within the first 2 to 4 weeks of life. In discusses the clinical management and prognosis newborn foals, periarticular laxity is the most of these postural deformities. likely cause, and these foals require no special treatment other than a short period of con- TREATMENT trolled exercise. In our opinion, mildly and The absence of controlled studies has moderately affected foals should not be confined impaired the accumulation of scientific data to a stall because exercise is important for nor- guiding the management of angular limb defor- mal muscular development and resolution of the mities in foals (Table 1). -

Genetic and Phenotypic Analysis of Elbow Dysplasia in Four Large Swedish Dog Breeds – an Evaluation of the Screening Programme and Clinical Symptoms
Genetic and phenotypic analysis of elbow dysplasia in four large Swedish dog breeds – an evaluation of the screening programme and clinical symptoms Genetisk och fenotypisk analys av armbågsdysplasi hos fyra storvuxna svenska hundraser – en evaluering av hälsoprogrammet och kliniska besvär Anna Medved Master Thesis • 30 hp Swedish University of Agricultural Sciences, SLU Department of Animal Breeding and Genetics Agriculture Programme – Animal Science Uppsala 2020 Genetic and phenotypic analysis of elbow dysplasia in four large Swedish dog breeds – an evaluation of the screening programme and clinical symptoms Genetisk och fenotypisk analys av armbågsdysplasi hos fyra storvuxna svenska hundraser – en evaluering av hälsoprogrammet och kliniska besvär Anna Medved Supervisor: Katja Nilsson, Swedish University of Agricultural Sciences, Department of Animal Breeding and Genetics Assistant supervisor: Sofia Malm, Geneticist at the Swedish Kennel Club Assistant supervisor: Nils Lundeheim, Swedish University of Agricultural Sciences, Department of Animal Breeding and Genetics Examiner: Erling Strandberg, Swedish University of Agricultural Sciences, Department of Animal Breeding and Genetics Credits: 30 hp Level: A2E Course title: Independent Project in Animal Science Course code: EX0872 Programme/education: Agriculture Programme – Animal Science Course coordinating dept: Place of publication: Uppsala Year of publication: 2020 Cover picture: Svenska Kennelklubben Keywords: elbow dysplasia, screening, fragmented coronoid process, osteochondritis dissecans, ununited anconeal process, elbow incongruity Swedish University of Agricultural Sciences Faculty of Veterinary Medicine and Animal Science (VH) Department of Animal Breeding and Genetics Archiving and publishing Approved students’ theses at SLU are published electronically. As a student, you have the copyright to your own work and need to approve the electronic publishing. When you have approved, metadata and full text of your thesis will be visible and searchable online. -

Conformational Limb Abnormalities and Corrective Farriery for Foals
Conformational Limb Abnormalities and Corrective Farriery for Foals For the past couple of years the general public has questioned the horse industry, and one of the questions is “Are we breeding horses more prone to breakdown with injuries”? It seems that we hear more often now that there are more leg and foot problems than ever before. Is it that we are more aware of conformational deficits and limb deviations, or are there some underlying factors that make our horses prone to injury? To improve, or even just maintain the breed, racing should prove soundness as well as speed and stamina. 1 The male side is usually removed from the bloodline if unable to produce good results at the racetrack. However, the female may have such poor conformation that she never even gets into training, but yet she enters the broodmare band. The generational effect of this must surely lead to an increase in the number of conformational deficits in our foals and yearlings.1 Something that we hear quite often is “Whoever saw the perfect horse”? and “There are plenty of horses with poor conformation that win races”. That doesn’t mean we should not continue to attempt to produce horses with good conformation. There is an acceptable range of deviation from the ideal, and therefore these deformities have to be accepted if it does not jeopardize the overall athletic soundness of the horse. Although mild conformational deficits may not significantly impact soundness, more significant limb deformities cause abnormal limb loading, lameness, gait abnormalities, and interference issues. 2 Developmental deformities of the limb include angular, flexural, and rotational limb deformities. -

Veterinary Orthopedic Society 43Rd Annual Conference Abstracts
A16 2016 VOS Abstracts Veterinary Orthopedic Society purpose of this study was to compare DI measurements with hip arthroscopy findings in dogs undergoing DPO. rd 43 Annual Conference Abstracts Materials and Methods: Medical records, arthroscopic images, and radio- graphs from 14 patients (26 hips) undergoing unilateral or bilateral DPO were reviewed. DI was measured using distraction view radiographs. Arthro- February 27 – March 5, 2016 scopic images were analyzed and findings graded using the Modified Outer- Big Sky, Montana, USA bridge Scale. ANOVA was used to compare DI between grade groups. Results: The highest grades of cartilage wear were noted cranially on the fe- moral head, mid-acetabulum, and caudally on the DAR. No significant dif- Part II ferences in DI were identified between groups of cartilage wear grade on the femoral head, acetabulum, or DAR, nor were any significant differences in DI 2016 VOS Abstracts VOS 2016 PODIUM ABSTRACTS (continued) identified between grade groups for the round ligament, labrum, or degree of synovitis. Discussion/Conclusion: This study found no significant differences in DI 52 COMPARISON OF ULTRASOUND AND MRI TO with different grades of cartilage, DAR, round ligament, or labral wear, or de- ARTHROSCOPY FOR ASSESSMENT OF MEDIAL MENISCAL gree of synovitis. No trend was noted for DI to increase as wear grade in- PATHOLOGY IN DOGS WITH CRANIAL CRUCIATE LIGAMENT creased in any region of the joints evaluated. Arthroscopic evaluation should DISEASE be considered prior to DPO as part of appropriate candidate selection. Acknowledgement: There was no proprietary interest or funding provided Samuel Patrick Franklin1; James L. Cook2; Cristi R. -

University of Washington Department of Orthopaedics and Sports Medicine
Discoveries 2012 University of Washington Department of Orthopaedics and Sports Medicine UNIVERSITY OF WASHINGTON Department of Orthopaedics and Sports Medicine 2012 Research Report Department of Orthopaedics and Sports Medicine University of Washington Seattle, WA 98195 Editor-in-ChiEf: Jens R. Chapman, M.D. Managing Editor: Fred Westerberg Front Cover Illustration: Pottery by Jack Routt, Photographer: Conrad Lilleness. The cover features a ceramic basin by Jack Routt. In Latin, the word for basin is sometimes translated as pelvis. Pelvic surgery is one of the orthopaedic specialities of Jack’s father, Milton L. Routt, Jr., M.D., Professor. Jack Routt (above) is a junior at King’s High School in Seattle, WA who enjoys creating ceramic art. Contents 1 Foreword 4 In Memoriam: Paul J. Benca, M.D. July 24, 1958 - June 27, 2011 6 David R. Eyre, Ph.D.: Steindler Award 7 Peter Simonian, M.D., 2012 Grateful Alumnus 8 Robert M. Berry, M.D., 2012 Distinguished Alumnus 9 New Faculty 10 Department of Orthopaedics and Sports Medicine Faculty 14 Visiting Lecturers 16 A Very Successful Year in Orthopaedics Salvage of Failed Custom Total Ankle 20 Michael E. Brage, M.D. Replacement: A Case Report Temporary External Fixation in Calcaneal 22 John Munz, M.D., Patricia A. Kramer, Ph.D., Fractures and Stephen K. Benirschke, M.D. Enhancing Pedicle Screw Fixation in the 23 Harsha Malempati, M.D., Bopha Chrea B.S., Lumbar Spine Utilizing Allograft Bone Plug Jeffrey Campbell M.S., Sonja Khan B.S., Interference Fixation: A Biomechanical Study Randal P. Ching, Ph.D., and Michael J. Lee, M.D. -

ANGULAR LIMB DEFORMITIES of FOALS in These Congenital Or Acquired Skeletal Defects, the Distal Portion of a Limb Deviates Laterally Or Medially Early in Neonatal Life
tions. The various types of tenosynovitis include idiopathic, acute, chronic, and septic 1 (infectious). Idiopathic synovitis refers to synovial distention of tendon sheaths in young f animals, in which the cause .is uncertain. Acute and chronic tenosynovitis are due to trauma Septic tenosynovitis may be associated with penetrating wounds, local exten- sion of infection, or a hematogenous infection. Clinical Findings and Diagnosis: There are varying degrees of synovial distention of the tendon sheath and lameness, depending on the severity. Horses are markedly lame in septic tenosynovitis. Chronic tenosynovitis is common in horses in the tarsal sheath of the hock (thoroughpin) and in the digital sheath (tendinous windpuffs). These two entities must be differentiated from bog spavin and synovial effusion of the fetlock. aeatment: In idiopathic cases, no treatment is initially recommended. Acute cases with clinical signs may be treated symptomatically with cold packs, nonsteroidal anti-in- flammatory drugs, and rest. Application of counterirritants and bandaging has been used in more chronic cases. Radiation therapy is helpful. Septic tenosynovitis requires systemic antibiotics and drainage. If adhesions develop between the tendon sheath and the tendon, persistent effusion and lameness is the rule. Congenital and inherited anomalies can result in the birth of diseased or deformed neonates. Congenital disorders can be due to viral infections of the fetus or to ingestion 1 of toxic plants by the dam at certain stages of gestation. The musculoskeletal system can also be affected by certain congenital neurologic disorders. See also WNGEHITAL MY- OPATHIES, p 867. ANGULAR LIMB DEFORMITIES OF FOALS In these congenital or acquired skeletal defects, the distal portion of a limb deviates laterally or medially early in neonatal life. -

Proceedings 10.Pdf
FRONTISPIECE. Steve Nesbitt was awarded the 4th L. H. WALKINSHAW CRANE CONSERVATION AwARD on 10 February 2006 in Zacatecas City, Zacatecas, Mexico. Steve’s work with Florida sandhill cranes began over 3 decades ago. He first published a paper on cranes in 1974, and since has authored or co-authored >65 publications on cranes. Steve, a founding member of the North American Crane Working Group, is the world’s authority on Florida sandhill cranes. Steve has been active in the conservation of other races of sandhill cranes, including the eastern greater sandhill crane and the Cuban sandhill crane. Over 27 years Steve banded 1,093 individual sandhill cranes. Steve was the driving force in Florida for the re-establishment of non-migratory whooping cranes. In addition, Steve has published 40 other papers on species such as red- cockaded woodpeckers and wood storks. His life’s work (much of which can only be described as of pioneering quality) focused on conservation of species threatened with extinction. Though employed for 34 years by the Florida Fish and Wildlife Conservation Commission (previously the Florida Game and Fresh Water Fish Commission), Steve’s conservation efforts go beyond Florida’s boundaries. Steve, through the donation/translocation from the State of Florida, has been instrumental in the recovery of the brown pelican and bald eagle. (Photo by Scott Hereford.) Front Cover: At first light in the Sierra Madre, sandhill cranes fly over pasture lands toward feeding grounds near Laguna de Babicora in the Chihuahuan Desert of northern Mexico. Image Copyright Michael Forsberg / www.michaelforsberg.com. Back Cover: Scenes from the Tenth Workshop in Zacatecas by Marty Folk.