University of Florida Thesis Or Dissertation Formatting
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
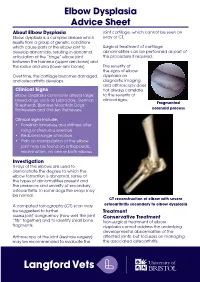
Elbow Dysplasia Advice Sheet About Elbow Dysplasia Joint Cartilage, Which Cannot Be Seen on Elbow Dysplasia Is a Complex Disease Which X-Ray Or CT
Elbow Dysplasia Advice Sheet About Elbow Dysplasia joint cartilage, which cannot be seen on Elbow dysplasia is a complex disease which x-ray or CT. results from a group of genetic conditions which cause parts of the elbow joint to Surgical treatment of cartilage develop abnormally, resulting in abnormal abnormalities can be performed as part of articulation of the “hinge” elbow joint this procedure if required. between the humerus (upper arm bone) and the radius and ulna (lower arm bones). The severity of the signs of elbow Over time, the cartilage becomes damaged, dysplasia on and osteoarthritis develops. diagnostic imaging and arthroscopy does Clinical Signs not always correlate Elbow dysplasia commonly affects large to the severity of breed dogs, such as Labradors, German clinical signs. Fragmented Shepherds, Bernese Mountain Dogs, Rottweilers and Golden Retrievers. coronoid process Clinical signs include; • Forelimb lameness and stiffness after rising or strenuous exercise • Reduced range of motion • Pain on manipulation of the elbow joint may be found on orthopaedic examination, on one or both elbows Investigation X-rays of the elbows are used to demonstrate the degree to which the elbow formation is abnormal, some of the types of abnormalities present and the presence and severity of secondary osteoarthritis. In some dogs the x-rays may be normal. CT reconstruction of elbow with severe A computed tomography (CT) scan may osteoarthritis secondary to elbow dysplasia be suggested to further Treatment assess joint congruency (how well the joint Conservative Treatment “fits” together) and to identify small bone Non-surgical treatment of elbow fragments. dysplasia cannot address the underlying developmental abnormalities of the Arthroscopy of the joint (keyhole surgery) affected joints, but focusses on managing may be recommended to evaluate the the associated osteoarthritis. -

Surgical Treatment of Canine Elbow Dysplasia (MCPD, UAP, OCD, EI)
PROCEEDINGS 33rd annual meeting of the INTERNATIONAL ELBOW WORKING GROUP September 24th 2018 WSAVA-FASAVA congress, Marina Bay Sands Congress Venue, Singapore. Welcome at the 33rd meeting of the International Elbow Working Group The International Elbow Working Group (IEWG) is an affiliate of the World Small Animal Veterinary Association (WSAVA). The board of the IEWG is therefore very grateful to the organisers of the 43rd WSAVA congress and the 9th FASAVA congress, in particular to Dr Shane Ryan, chairman of the Congress Committee, and Dr Frédéric Gaschen, chairman of the Congress Scientific Program Committee to be invited and facilitated in organizing a seminar during the pre-congress meeting of the WSAVA-2018 congress. By this invitation, the WSAVA underpins the importance of the role of the IEWG to perform the task of exchanging important information to the world veterinary community regarding the causes, prevalence, screening, therapy and prevention of one of the most important non-traumatic diseases of the locomotion system in dogs. The IEWG fulfils this task by organizing annual meetings with the help of esteemed scientist in the field of genetics, radiology, orthopedic surgery, and related fields to present the newest science and overviews on Elbow Dysplasia. Earlier this month, the IEWG had a seminar at the World Veterinary Orthopedic Congress, a joined meeting of the European Society of Veterinary Orthopedics and Traumatology (ESVOT) and the American Veterinary Orthopedic Society (VOS), with participants of European countries and the America’s. The IEWG is happy to be able to share new information with participants of the Asian and other countries coming together in Singapore. -

Synovial Joints Permit Movements of the Skeleton
8 Joints Lecture Presentation by Lori Garrett © 2018 Pearson Education, Inc. Section 1: Joint Structure and Movement Learning Outcomes 8.1 Contrast the major categories of joints, and explain the relationship between structure and function for each category. 8.2 Describe the basic structure of a synovial joint, and describe common accessory structures and their functions. 8.3 Describe how the anatomical and functional properties of synovial joints permit movements of the skeleton. © 2018 Pearson Education, Inc. Section 1: Joint Structure and Movement Learning Outcomes (continued) 8.4 Describe flexion/extension, abduction/ adduction, and circumduction movements of the skeleton. 8.5 Describe rotational and special movements of the skeleton. © 2018 Pearson Education, Inc. Module 8.1: Joints are classified according to structure and movement Joints, or articulations . Locations where two or more bones meet . Only points at which movements of bones can occur • Joints allow mobility while preserving bone strength • Amount of movement allowed is determined by anatomical structure . Categorized • Functionally by amount of motion allowed, or range of motion (ROM) • Structurally by anatomical organization © 2018 Pearson Education, Inc. Module 8.1: Joint classification Functional classification of joints . Synarthrosis (syn-, together + arthrosis, joint) • No movement allowed • Extremely strong . Amphiarthrosis (amphi-, on both sides) • Little movement allowed (more than synarthrosis) • Much stronger than diarthrosis • Articulating bones connected by collagen fibers or cartilage . Diarthrosis (dia-, through) • Freely movable © 2018 Pearson Education, Inc. Module 8.1: Joint classification Structural classification of joints . Fibrous • Suture (sutura, a sewing together) – Synarthrotic joint connected by dense fibrous connective tissue – Located between bones of the skull • Gomphosis (gomphos, bolt) – Synarthrotic joint binding teeth to bony sockets in maxillae and mandible © 2018 Pearson Education, Inc. -

Sokoto Journal of Veterinary Sciences Preliminary Evaluation Of
Sokoto Journal of Veterinary Sciences, Volume 17 (Number 2). June, 2019 RESEARCH ARTICLE Sokoto Journal of Veterinary Sciences (P-ISSN 1595-093X: E-ISSN 2315-6201) http://dx.doi.org/10.4314/sokjvs.v17i2.6 Ajadi & Doyin-Dada./Sokoto Journal of Veterinary Sciences, 17(2): 45 - 53. Preliminary evaluation of prevalence of hip and elbow dysplasia in Boerboel dogs RA Ajadi *& OA Doyin-Dada Department of Veterinary Medicine and Surgery, Federal University of Agriculture, Abeokuta PMB 2240, Alabata Road, Abeokuta, Ogun State, Nigeria *Correspondence: Tel.: +2347033800326; E-mail: [email protected] Copyright: © 2019 Abstract Ajadi & Doyin-Dada. Hip dysplasia (HD) and elbow dysplasia (ED) are developmental diseases that affect This is an open-access large breed of dogs disproportionately. Despite the large size of Boerboel dogs, there article published under are no breed prevalence for HD and ED in Nigeria. This study provides preliminary the terms of the information about HD and ED prevalence in Boerboels. Twenty Boerboels of both Creative Commons sexes were evaluated. Ventrodorsal radiographs of the hip joint and flexed lateral Attribution License radiographs of elbow joint were made from each dog, using digital technology. Hip which permits grading was done using the Fédération Cynologique Internationale system (World unrestricted use, Canine Organization), assigning grades ranging from A - E. Elbow radiographs were distribution, and graded based on the International Elbow Working Group criteria, and scores ranging reproduction in any from 0-3 were assigned. Prevalence of HD and ED were expressed as percentages. medium, provided the original author and Age and sex difference were compared using a chi square test. -

Elbow Dysplasia in Dogs
Elbow dysplasia in dogs Revised by Gary Clayton Jones BVetMed DSAO DVR FRCVS with additional graphics by Jonathan Clayton Jones The British Veterinary Association and the Kennel Club — working together for excellence in canine health Elbow dysplasia has been identified as a significant problem in many breeds. Importantly, the condition appears to be increasing worldwide. It begins in puppyhood, and can affect the dog for the rest of its life The ‘flexed mediolateral’ view is a side-on view of the elbow. This view allows examination of the secondary changes in ED which occur in the shaded areas. Note how some of the shaded areas here are overlaid by other structures, which makes them difficult to examine eterinary surgeons have been Once the dog reaches skeletal maturity disease in that they have primary lesions aware for many years of a number the primary lesions may stabilise. However, or osteoarthritis in their elbows but do not of conditions that begin in puppies once abnormal development has started appear obviously lame. Some dogs will be and cause lameness. Hip dysplasia with a primary lesion, further secondary symmetrically lame in each foreleg, which Vwas the first such disease to be widely changes follow, in particular, abnormal wear can be very difficult to see. Fortunately, these recognised and a scheme for its assessment of the joint surfaces and osteoarthritis subclinical dogs can often be identified by and control is well established in the UK. (sometimes termed arthrosis, or degenerative taking radiographs (X-ray images) of their Elbow dysplasia (ED) is a significant problem joint disease — DJD). -

Proceedings 2012
PROCEEDINGS 27th annual meeting of the INTERNATIONAL ELBOW WORKING GROUP April 11th 2012 ICC, hall 7b Birmingham, UK The International Elbow Working Group acknowledges the financial support by HILL’S PET NUTRITION 27th annual meeting IEWG, Birmingham UK, April 11th 2012, p 2 WELCOME ADDRESS Birmingham, April 2012 Dear IEWG-congress participant, The International Elbow Working Group (IEWG) has been founded by a group of veterinarians and dog breeders in Davis, CA, U.S.A. in 1989 with the aim to increase the knowledge on and awareness of elbow disease in dogs, and to support all stakeholders in disseminating new knowledge in this field. The interest in hereditary aspects of elbow dysplasia (ED) has been increasing ever since both among breeders and veterinary surgeons and radiologists alike. ED has been recognized as a cause of lameness in a large variety of dog breeds with an incidence as high as 50% of the screened dogs. Published data on prevalence vary depending on breed and country. These discrepancies can be a matter of screening bias (e.g. not including dogs suffering from the disease and treated at a young age, or not submitting films of affected dogs for official screening), of improvement gained by the implementation of breeding restrictions for affected dogs, or may be caused by different screening or grading modes. The latter emphasizes the necessity of uniform grading, preferably by the protocol introduced and refined by IEWG and in use in many countries for many years. Some research groups are in a process of performing molecular genetic research in the field of canine elbow dysplasia, to use the results in the future for screening of potential breeding stock. -

Genetic and Phenotypic Analysis of Elbow Dysplasia in Four Large Swedish Dog Breeds – an Evaluation of the Screening Programme and Clinical Symptoms
Genetic and phenotypic analysis of elbow dysplasia in four large Swedish dog breeds – an evaluation of the screening programme and clinical symptoms Genetisk och fenotypisk analys av armbågsdysplasi hos fyra storvuxna svenska hundraser – en evaluering av hälsoprogrammet och kliniska besvär Anna Medved Master Thesis • 30 hp Swedish University of Agricultural Sciences, SLU Department of Animal Breeding and Genetics Agriculture Programme – Animal Science Uppsala 2020 Genetic and phenotypic analysis of elbow dysplasia in four large Swedish dog breeds – an evaluation of the screening programme and clinical symptoms Genetisk och fenotypisk analys av armbågsdysplasi hos fyra storvuxna svenska hundraser – en evaluering av hälsoprogrammet och kliniska besvär Anna Medved Supervisor: Katja Nilsson, Swedish University of Agricultural Sciences, Department of Animal Breeding and Genetics Assistant supervisor: Sofia Malm, Geneticist at the Swedish Kennel Club Assistant supervisor: Nils Lundeheim, Swedish University of Agricultural Sciences, Department of Animal Breeding and Genetics Examiner: Erling Strandberg, Swedish University of Agricultural Sciences, Department of Animal Breeding and Genetics Credits: 30 hp Level: A2E Course title: Independent Project in Animal Science Course code: EX0872 Programme/education: Agriculture Programme – Animal Science Course coordinating dept: Place of publication: Uppsala Year of publication: 2020 Cover picture: Svenska Kennelklubben Keywords: elbow dysplasia, screening, fragmented coronoid process, osteochondritis dissecans, ununited anconeal process, elbow incongruity Swedish University of Agricultural Sciences Faculty of Veterinary Medicine and Animal Science (VH) Department of Animal Breeding and Genetics Archiving and publishing Approved students’ theses at SLU are published electronically. As a student, you have the copyright to your own work and need to approve the electronic publishing. When you have approved, metadata and full text of your thesis will be visible and searchable online. -
Orthopaedics Instructions: to Best Navigate the List, First Download This PDF File to Your Computer
Orthopaedics Instructions: To best navigate the list, first download this PDF file to your computer. Then navigate the document using the bookmarks feature in the left column. The bookmarks expand and collapse. Finally, ensure that you look at the top of each category and work down to review notes or specific instructions. Bookmarks: Bookmarks: notes or specific with expandable instructions and collapsible topics As you start using the codes, it is recommended that you also check in Index and Tabular lists to ensure there is not a code with more specificity or a different code that may be more appropriate for your patient. Copyright APTA 2016, ALL RIGHTS RESERVED. Last Updated: 09/14/16 Contact: [email protected] Orthopaedics Disorder by site: Ankle Achilles tendinopathy ** Achilles tendinopathy is not listed in ICD10 M76.6 Achilles tendinitis Achilles bursitis M76.61 Achilles tendinitis, right leg M76.62 Achilles tendinitis, left leg ** Tendinosis is not listed in ICD10 M76.89 Other specified enthesopathies of lower limb, excluding foot M76.891 Other specified enthesopathies of right lower limb, excluding foot M76.892 Other specified enthesopathies of left lower limb, excluding foot Posterior tibialis dysfunction **Posterior Tibial Tendon Dysfunction (PTTD) is not listed in ICD10 M76.82 Posterior tibial tendinitis M76.821 Posterior tibial tendinitis, right leg M76.822 Posterior tibial tendinitis, left leg M76.89 Other specified enthesopathies of lower limb, excluding foot M76.891 Other specified enthesopathies of right lower limb, -

Australian Cattle Dog Health Testing
AUSTRALIAN CATTLE DOG HEALTH TESTING “BREEDER OF HEART” REQUIREMENTS All purebred or mixed breed dogs can have genetic and other health issues. Testing the sire and dam before breeding is a way to decrease the frequency of or prevent disorders. Your breeder should be willing and able to discuss health and genetic issues found in your chosen breed. Your breeder should also be willing to show you the proof of testing. They should be able to verify which dogs the tests relate to through means such as microchips or tattoos that correspond to the test results. They should be willing to give you copies of tests related to your new dog. Look at what the actual test results say and do your research at sites like http://www.offa.org (Orthopedic Foundation for Animals) and http://www.acdhew.org (Australian Cattle Dog Health, Education and Welfare) to understand what they mean. Even if your breeder does all suggested testing, health challenges may still arise as these are living creatures, not machines. However, as a result of testing the parents and careful breeding based on testing, the likelihood of getting a healthy puppy is higher. Testing should be done on the sire and dam before breeding as described below. Some tests can also be performed on puppies, as noted. On DNA tests, the point is to avoid producing “affected” dogs. “Carriers” of disorders can make perfectly fine pets but should only be bred to “clear” dogs to avoid producing more “affected” dogs. Sometimes the breeder has done generations of tests to produce “obligate” clear dogs so the puppies themselves are no longer tested for certain disorders. -

(Bucked Shins) in the Flat Racing Horse: Prevalence, Diagnosis, Pathogenesis, and Associated Factors
Journal of Dairy, Veterinary & Animal Research Mini Review Open Access A review of dorsal metacarpal disease (bucked shins) in the flat racing horse: prevalence, diagnosis, pathogenesis, and associated factors Abstract Volume 5 Issue 6 - 2017 Dorsal metacarpal disease (DMD) is the most common cause of lostdays to training S Couch,1 BD Nielsen2 and racing in Thoroughbred racehorses. Colloquially termed ‘bucked’ or ‘sore’ shins, 1Royal (Dick) School of Veterinary Studies, University of this initially painful condition commonly occurs in the first season of training and can Edinburgh, United Kingdom raise welfare concerns. Clinical signs include pain with digital palpation and swelling 2Department of Animal Science, Michigan State University, USA on the dorsal, and sometimes dorso-medial, aspect of the third metacarpal (McIII). Periostitis and excessive growth of periosteal bone can be present as a response to Correspondence: Brian D Nielsen, Michigan State University, high strain cyclic fatigue. Whilst DMD can resolve with rest or reduced exercise, it Department of Animal Science, 474 S. Shaw Lane, East Lansing, can leave bone susceptible to future catastrophic fracture at the same site, particularly MI 48824 1225, USA, Tel 517 432 1378, Fax 517 353 1699, saucer fractures of the lamellar bone of the diaphysis. Some trainers continue to work Email [email protected] an animal through DMD, with the view that it will only happen once, but it can re- occur. Additionally, the animal is in discomfort and has a weakened skeletal system. Received: September 13, 2017 | Published: September 25, In vivo studies of the effects of cyclic strain on the skeletal system of Thoroughbreds 2017 are notoriously difficult, due to the many variables involved and in vitro studies cannot mimic true training and racing conditions. -

Clinical Medical Policy
CLINICAL MEDICAL POLICY Noninvasive Electrical Bone Growth Stimulators Policy Name: (osteogenesis stimulators) Policy Number: MP-070-MD-PA Responsible Department(s): Medical Management Provider Notice Date: 12/15/2018 Issue Date: 01/15/2019 Effective Date: 01/15/2019 Annual Approval Date: 10/17/2019 Revision Date: N/A Products: Gateway Health℠ Medicaid Application: All participating hospitals and providers Page Number(s): 1 of 78 DISCLAIMER Gateway Health℠ (Gateway) medical policy is intended to serve only as a general reference resource regarding coverage for the services described. This policy does not constitute medical advice and is not intended to govern or otherwise influence medical decisions. POLICY STATEMENT Gateway Health℠ may provide coverage under the medical-surgical and DME benefits of the Company’s Medicaid products for medically necessary noninvasive electrical bone growth stimulators as treatment of nonunion long bone fractures or congenital pseudarthrosis. This policy is designed to address medical necessity guidelines that are appropriate for the majority of individuals with a particular disease, illness or condition. Each person’s unique clinical circumstances warrant individual consideration, based upon review of applicable medical records. (Current applicable Pennsylvania HealthChoices Agreement Section V. Program Requirements, B. Prior Authorization of Services, 1. General Prior Authorization Requirements.) Policy No. MP-070-MD-PA Page 1 of 78 DEFINITIONS Prior Authorization Review Panel - A panel of representatives from within the PA Department of Human Services who have been assigned organizational responsibility for the review, approval and denial of all PH-MCO Prior Authorization policies and procedures. Non-invasive (Osteogenic) Electrical Bone Growth Stimulator – A device that uses pulsed- electromagnetic fields, capacitative coupling or combined magnetic fields to generate a weak electric current through the target site. -

Simultaneous Dislocation of the Radial Head and Distal Radio-Ulnar Joint
Jin et al. BMC Surgery (2020) 20:71 https://doi.org/10.1186/s12893-020-00717-8 CASE REPORT Open Access Simultaneous dislocation of the radial head and distal radio-ulnar joint without fracture in an adult patient: a case report and review of literature Xiang-Yun Jin1† , Wen-Bo Zhao1† , Yu-Qi Dong1* and Yi-Gang Huang2* Abstract Background: Simultaneous dislocation of the radial head and distal radio-ulnar joint without fracture (Criss-Cross Injury) in an adult patient is rarely reported in previous studies. The pathological changes and injury patterns have not been clearly demonstrated. Case presentation: A 26-year-old woman presented with acute pain of the right wrist and elbow after a fall from cycling. Physical examination revealed an unstable elbow and wrist joint. Plain radiographs showed volar dislocation of the radial head and dorsal dislocation of the distal radius without associated fracture, forming a criss- cross appearance of the ulna and radius on the lateral radiograph. MRI images confirmed partial rupture of the proximal interosseous membrane from its dorsal attachment on the radius, as well as partial rupture of the medial collateral ligament. Conservative treatment failed because the radiocapitellar joint and distal radio-ulnar joint could not be simultaneously reduced. Surgical exploration revealed a highly unstable radial head, but the annular ligament was found to be intact. Manual force was applied to reduce the radial head and a percutaneous K-wire was used to stabilize the proximal radioulnar joint with the forearm in full supination. After surgery, the elbow was immobilized in 90° flexion by a long arm cast for 4 weeks.