Poultry Genetics -Mutations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
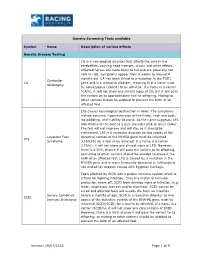
19/07/2018 Page 1 of 9 Genetic Screening Tests Available Symbol
Genetic Screening Tests available Symbol Name Description of variant effects Genetic Disease Testing CA is a neurological disorder that affects the cells in the cerebellum, causing head tremors, ataxia and other effects. Affected horses are more likely to fall and are generally not safe to ride. Symptoms appear from 6 weeks to around 4 months old. CA has been linked to a mutation in the TOE1 Cerebellar CA gene and is a recessive disorder, meaning that a horse must Abiotrophy be homozygous (CA/CA) to be affected. If a horse is a carrier (CA/n), it will not show any clinical signs of CA, but it will pass the variant on to approximately half its offspring. Mating to other carriers should be avoided to prevent the birth of an affected foal. LFS causes neurological dysfunction in foals. The symptoms include seizures, hyperextension of the limbs, neck and back, leg paddling, and inability to stand. As the name suggests, LFS also dilutes to the coat to a pale lavender pink or silver colour. The foal will not improve and will die, so it should be euthanised. LFS is a recessive disorder so two copies of the Lavender Foal defective version of the MYO5A gene must be inherited LFS Syndrome (LFS/LFS) for a foal to be affected. If a horse is a carrier (LFS/n), it will not show any clinical signs of LFS. However, there is a 50% chance it will pass the variant to its offspring, so mating to other carriers should be avoided to prevent the birth of an affected foal. -

Horse Coat Color Test Results Dt34839
HORSE COAT COLOR TEST RESULTS TONI PERDEW Case: DT34839 3005 LEXINGTON CT Date Received: 05-Aug-2013 BEDFORD, IA 50833 Report Date: 07-Aug-2013 Report ID: 3263-6964-9709-4059 Verify report at https://www.vgl.ucdavis.edu/myvgl/verify.html Horse: HI JACD SILVER Reg: 5220615 DOB: 05/31/2009 Breed: QH Sex:M Alt. ID: Sire: MAINLY MERLIN Reg: Dam: BAILEYS BADLAND BUCK Reg: W10 DOMINANT RED FACTOR Not requested. Not requested. WHITE SPLASHED AGOUTI Not requested. Not requested. WHITE CREAM Not requested. TOBIANO Not requested. PEARL Not requested. LEOPARD Not requested. SILVER Not requested. GRAY Not requested. Horse is homozygous for the Dun gene. All DUN ROAN Not requested. D/D offspring should be dun dilute. CHAMPAGNE Not requested. LETHAL WHITE Not requested. OVERO SABINO 1 Not requested. For more details on horse coat color tests, please visit: www.vgl.ucdavis.edu/services/coatcolorhorse.php Tests for Gray, Leopard/Appalossa and Lethal White Overo are performed under license. Horse Coat Color Results with Explanations Red Factor Silver e/e - Only the red factor detected. Basic color is sorrel or chestnut in the absence of other N/N - No evidence of the altered sequence detected. modifying genes. N/Z - One copy of the altered sequence detected. Black-based horses will be chocolate with flaxen E/e - Both black and red factors detected. Either E or e transmitted to offspring. Basic color is or lightened mane and tail. Bay-based horses will have lightened black pigment on lower legs, mane black, bay or brown in the absence of other modifying genes. -

Take a Virtual Tour of Cackle Hatchery® 1999-2009
Nancy Smith (Owner) "We thank you for all your past business and look forward to serving you in the future." Our phone call center is very busy taking your orders and securing shipping dates for you. We try to give you as much time as you need to answer all your questions. “So give us a call!” Our pure breed hatching eggs in cases coming in from our breeder farms by the truck load... by the van load.... by the trailer load.... brought in every seven days to Cackle Hatchery. Three incubators in the background. We have 44 total incubators at Cackle Hatchery. Egg Varieties: Brown Eggs - White Rock; White Eggs - Brown Leghorn; Green/blue/pink eggs- Ameraucana. Eggs are set two times a week to produce two large hatching days a week. Chicken eggs take 21 days to hatch (18 days in incubator, 3 days in hatcher). Trayed eggs in racks ready to be set into the incubators. Clifton Smith (Owner), making modifications to temperature and humidity on a Natureform Incubator. Trayed eggs in racks ready to be set into Vintage Robbins Incubators. 1950's Robbins incubator. One of 44 total incubator racks in use. 1980's Natureform Incubator. One of 44 total incubator racks in use. 1980's Natureform Incubator. One of 44 total incubator racks in use. Eggs are set every Monday and Wednesday. Racks are always kept at full capacity. Most trays hold 180 eggs. There is a front tray and back tray to the incubator rack. An incubator rack holds about 12,500 eggs. Checking the automatic rack turner is important. -

( 12 ) Patent Application Publication ( 10 ) Pub . No . : US 2019 / 0029236 A1
US 20190029236A1 ( 19) United States (12 ) Patent Application Publication (10 ) Pub. No. : US 2019 /0029236 A1 OFFEN ( 43 ) Pub . Date : Jan . 31 , 2019 ( 54 ) METHODS FOR GENDER DETERMINATION C12Q 1/ 6879 (2006 .01 ) OF AVIAN EMBRYOS IN UNHATCHED C12N 15 /90 ( 2006 . 01 ) EGGS AND MEANS THEREOF C12N 9 /02 ( 2006 .01 ) (52 ) U . S . CI. ( 71) Applicant: EGGXYT LTD , Jerusalem (IL ) CPC .. A01K 67 / 0275 (2013 .01 ) ; C12N 15 / 102 ( 2013 .01 ) ; C120 1 /6879 ( 2013 . 01 ) ; A01K ( 72 ) Inventor: Daniel OFFEN , Kfar HaRoe ( IL ) 2227/ 30 ( 2013 .01 ) ; C12N 9 /0069 ( 2013. 01 ) ; A01K 2217 /07 ( 2013 .01 ) ; C12N 15 / 907 ( 21 ) Appl. No. : 15 /996 ,045 ( 2013 .01 ) (22 ) Filed : Jun . 1 , 2018 Related U . S . Application Data (57 ) ABSTRACT (63 ) Continuation - in - part of application No . PCT / IL2016 / The present invention relates to methods of gender deter 051291 , filed on Dec . 1 , 2016 . mination and identification in avian subjects . More specifi ( 60 ) Provisional application No . 62/ 262 ,409 , filed on Dec . cally , the invention provides non - invasive methods using 3 , 2015 . transgenic avian animals that comprise at least one reporter gene integrated into at least one gender chromosome Zor W . Publication Classification The transgenic avian animals of the invention are used for (51 ) Int . Ci. gender determination and selection of embryos in unhatched A01K 677027 ( 2006 . 01 ) avian eggs . C12N 15 / 10 ( 2006 .01 ) Specification includes a Sequence Listing . KYYYYYYYYYY Patent Application Publication Jan . 31 , 2019 Sheet 1 of 3 US 2019 / 0029236 A1 ** BALISERE* SSSSSSS Fig . 1A TXXXXXXXXXA Fig . 1B Fig . 2A Patent Application Publication Jan . -

Color Coat Genetics
Color CAMERoatICAN ≤UARTER Genet HORSE ics Sorrel Chestnut Bay Brown Black Palomino Buckskin Cremello Perlino Red Dun Dun Grullo Red Roan Bay Roan Blue Roan Gray SORREL WHAT ARE THE COLOR GENETICS OF A SORREL? Like CHESTNUT, a SORREL carries TWO copies of the RED gene only (or rather, non-BLACK) meaning it allows for the color RED only. SORREL possesses no other color genes, including BLACK, regardless of parentage. It is completely recessive to all other coat colors. When breeding with a SORREL, any color other than SORREL will come exclusively from the other parent. A SORREL or CHESTNUT bred to a SORREL or CHESTNUT will yield SORREL or CHESTNUT 100 percent of the time. SORREL and CHESTNUT are the most common colors in American Quarter Horses. WHAT DOES A SORREL LOOK LIKE? The most common appearance of SORREL is a red body with a red mane and tail with no black points. But the SORREL can have variations of both body color and mane and tail color, both areas having a base of red. The mature body may be a bright red, deep red, or a darker red appearing almost as CHESTNUT, and any variation in between. The mane and tail are usually the same color as the body but may be blonde or flaxen. In fact, a light SORREL with a blonde or flaxen mane and tail may closely resemble (and is often confused with) a PALOMINO, and if a dorsal stripe is present (which a SORREL may have), it may be confused with a RED DUN. -

Sexing Day-Old Chicks on Small and Backyard Flocks
eXtension Sexing Day-Old Chicks on Small and Backyard Flocks articles.extension.org/pages/65437/sexing-day-old-chicks-on-small-and-backyard-flocks Written by: Dr. Jacquie Jacob, University of Kentucky Figure 1. Genetic basis for sex determination in mammals and birds With most animals, it is relatively easy to determine the sex of the newborn. The male reproductive organs are located on the outside of the body and are relatively easy to see, even in newborns. This is not the case with poultry. In male birds, the reproductive organs are inside the body cavity. This makes sexing newly hatched chicks difficult. There are two methods of sexing chicks that can be used at hatcheries: vent sexing and feather sexing. Vent sexing was developed in Japan and brought to North American poultry producers in the 1930s. Vent sexing is a skill that takes a long time to develop. It involves holding the chick upside down in one hand, expelling the fecal material, and everting (turning outward) the vent area. The producer can then look for the presence or absence of a rudimentary male sex organ. This process sounds much easier than it actually is. To accurately sex chicks in this way, you need to be well trained and to have had a great deal of practice. There are very few schools that still teach chick sexing. Feather sexing is possible for some chicken breeds. The Rhode Island Red and New Hampshire breeds can be sexed by wing color at hatching. Male chicks have a white spot on the down over the wing web. -

EQUINE COAT COLORS and GENETICS by Erika Eckstrom
EQUINE COAT COLORS AND GENETICS By Erika Eckstrom Crème Genetics The cream gene is an incomplete dominant. Horse shows a diluted body color to pinkish-red, yellow-red, yellow or mouse gray. The crème gene works in an additive effect, making a horse carrying two copies of the gene more diluted towards a crème color than a horse with one copy of the gene. Crème genes dilute red coloration more easily than black. No Crème Genes One Crème Gene Two Crème Genes Black Smokey Black Smokey Crème A Black based horse with no "bay" A Black horse that received one copy A Black horse that received one copy gene, and no dilution gene, ranging of the crème dilution gene from one of the crème gene from both of its from "true" black to brown in of its parents, but probably looks no parents, possessing pink skin, blue eyes, and an orange or red cast to the appearance. different than any other black or brown horse. entire hair coat. Bay Buckskin Perlino A Black based horse with the "bay" Agouti gene, which restricts the A Bay horse that received one copy A Bay horse that received one copy of black to the mane, tail and legs of the crème dilution gene from its the crème gene from both of its (also called black "points") and no parents, giving it a diluted hair coat parents, and has pink skin, blue eyes, a ranging in color from pale cream, cream to white colored coat and a dilution gene. gold or dark "smutty" color, and has darker mane and tail (often orange or black "points". -

Features of Coat Color and Markings and Impact of Dun Factor on Vyatka Horse Breed
BIO Web of Conferences 17, 00202 (2020) https://doi.org/10.1051/bioconf/20201700202 FIES 2019 Features of coat color and markings and impact of dun factor on Vyatka horse breed Natalia F. Belousova1, Svetlana P. Bass2, Svetlana A. Zinoveva3, Sergei A. Kozlov3,*, and Sergei S. Markin3 1All-Russian Research Institute of Horse Breeding (VNIIK), 391105 Divovo Village, Ryazan Region, Russia 2Izhevsk State Agricultural Academy, 426069 Izhevsk, Russia 3Moscow State Academy of Veterinary Medicine and Biotechnology – MVA named after K.I. Skryabin, 109472 Moscow, Russia Abstract. The predominant coat colors in Vyatka horse breed are bay-brown (69.6 %) and mousey (20.8 %). Among the genotyped livestock, three genotypes of the base bay coat color (EE/AA, EE/Aa, Ee/AA) and two genotypes of the base solid blackcock (EE/a/a, Ee/aa) have been detected. The proportion of horses with Cr allele is 2.1 %. In Vyatka horse breed, three isabelline-brown horses (Cr/Cr) have been recorded and the presence of W20n allele was detected. Among the horses genotyped, 35.5 % are DD homozygous, 61.3 % are heterozygous (Dd1, Dd2), 3.2 % have the nd2/nd2 genotype. Allele d2 against the background of D does not always cause the presence of “wild” markings, unlike D/D. The influence of Dun-factor on the depigmentation area has not been detected. 39.9 % of horses have white markings (including 30 % of stallions), which are mainly facial markings (59.8 %), less often they are leg markings (21.6 %) or both facial and leg markings (18, 6 %). 1 Introduction At the same time, the chestnut (red and brown) coat color has become rather rare in the breed. -

International Revîëw of Poultry Science
TOME IX. 1936. No. 1/2 INTERNATIONAL REVÎËW OF POULTRY SCIENCE OFFICIAL ORGAN OF THE WORLD'S POULTRY SCIENCE ASSOCIATION ¡f73S EDITOR: Dr. B. J. C. TE HENNEPE ROTTERDAM (Holland) This Review is free fo all members of the World's Poultry Science Association. AH subscriptions should be sent to the Editor: Dr. B. J. C. te Hennepe, Rotterdam, or to the Secretary- Treasurer: Dr. G. F. Heuser, Cornell University, Ithaca, N.y., U.S.A. SUBSCRIPTIONS. $5.00 annually in advance. The personal membership of the W.P.S.A. per amounts to $5.00 year For affiliated societies „ „ $25.00 ADVERTISEMENT RATES. One page, per issue $12.00 Half page, per issue $7.00 NATIONAL POULTRY COUNCIL LAYING TRIALS REGISTER Vol. IX Containing Official Records obtained at RECOGNISED LAYING TRIALS Now on sale: Price 6d., postage paid. Copies of Vols. I-VIII are also on sale, price 6d. each, postage paid. Apply to: THE SECRETARY, National Poultry Council Avenue Chambers, 4, Vernon Place, London W. C. 1 ENGLAND Sir Edward Brown Above: Reidisminisfer for Agriculture and ßeidisbauernführer R. Walther Darré, President of the VI fh World's Poultry Congress. Left: Mr. Karl Vetter, Inspector General of the „Reichsnährstand" and President of the National Union of German Small Stock Breeders, Acting President of the VI th World's Poultry Congress. Below: Dr.Walther Kupsdi, ¡Secretary General of theVIfh World's Poultry Congress. Äf the reception of the Press in connection with the VI th World's Poultry Congress. The managing President of the Congress, Karl Vetter, explains to the Honorary President of the W.P.S.A., Sir Edward Brown, as well as to Ministerialdirektor Dr. -

Demand Driven Productivity in the Egg Business
Demand Driven Productivity in the Egg Business Prof. Dr. Dietmar K. Flock Dr. Flock and Dr. Havenstein came from Iowa State University (Animal Bree- ding) and the University of Wisconsin (Poultry Science) when they first met as colleagues at Heisdorf & Nelson Farms, where they were introduced to mo- dern poultry breeding. The authors have followed developments in different parts of the world for more than 50 years and review how progress in genetic potential was combined with improved labor efficiency, disease control and nutrition to produce eggs at least cost for the changing global demand. To contact the author: Mail at [email protected] Demand Driven Productivity in the Egg Business: Combining Advances in Genetics, Health Control and Nutrition to Meet Changing Consumer Preferences Abstract feed efficiency and carcass value in meat- poultry diseases more effectively, based Since seven decades, poultry meat and type chickens, while heterosis effects were on diagnostics, eradication and prophyl- egg consumption in many countries has exploited to maximize egg production, actic vaccination. Modern poultry nutriti- been accelerating at a faster rate than feed efficiency and egg quality in egg- on is based on least cost feed formulation global population growth. In this review, type chickens. A small number of primary for individual flocks to minimize feed cost we will focus on key contributions of ap- poultry breeders continue to improve the per egg in layer stock, and to minimize plied science and technology which exp- genetic potential for efficient production feed cost per unit of meat produced in lain the efficiency of today’s production: of poultry meat and eggs and supply li- broilers, making best possible use of lo- the change from hatch seasons and egg censed hatcheries in many countries with cally available resources, while minimizing production in open houses to hatching parent stock to multiply the improved ef- the environmental impact of production. -

Genetics and Genomics of Mammalian Pigment Patterns
GENETICS AND GENOMICS OF MAMMALIAN PIGMENT PATTERNS A DISSERTATION SUBMITTED TO THE DEPARTMENT OF GENETICS AND THE COMMITTEE ON GRADUATE STUDIES OF STANFORD UNIVERSITY IN PARTIAL FUFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY Lewis Zuocheng Hong August 2011 © 2011 by Zuocheng Lewis Hong. All Rights Reserved. Re-distributed by Stanford University under license with the author. This work is licensed under a Creative Commons Attribution- Noncommercial 3.0 United States License. http://creativecommons.org/licenses/by-nc/3.0/us/ This dissertation is online at: http://purl.stanford.edu/jx191nt1141 ii I certify that I have read this dissertation and that, in my opinion, it is fully adequate in scope and quality as a dissertation for the degree of Doctor of Philosophy. Gregory Barsh, Primary Adviser I certify that I have read this dissertation and that, in my opinion, it is fully adequate in scope and quality as a dissertation for the degree of Doctor of Philosophy. Andrew Fire I certify that I have read this dissertation and that, in my opinion, it is fully adequate in scope and quality as a dissertation for the degree of Doctor of Philosophy. David Kingsley I certify that I have read this dissertation and that, in my opinion, it is fully adequate in scope and quality as a dissertation for the degree of Doctor of Philosophy. Arend Sidow Approved for the Stanford University Committee on Graduate Studies. Patricia J. Gumport, Vice Provost Graduate Education This signature page was generated electronically upon submission of this dissertation in electronic format. An original signed hard copy of the signature page is on file in University Archives. -

Coat Color Testing Application
American Morgan Horse Association, Inc. 4037 Iron Works Parkway, Suite 130, Lexington, KY 40511-8508 COAT COLOR TESTING (802) 985-4944 • Fax: (859) 287-3555 [email protected] APPLICATION www.morganhorse.com COAT COLOR TESTING IS OPTIONAL. If the horse being tested tests positive for a specific coat color gene, that information can be recorded on the horse’s registration certificate (a $25 printing fee applies). Horse’s Name: _____________________________________________________________________________________________________________________________________________ Registration Number: ____________________________________________________________________ AVAILABLE COAT COLOR TESTS FEES (check appropriate box) (See reverse for descriptions) Member Non-Member q Cream Dilution q Gray First Coat Color Test .............................................................................................. $40 o $125 o q Red Factor and Agouti q Splash Each additional Coat Color Test on same horse ................................................ $25 o $110 o RUSH FEE (charge per horse) ............................................................................$100 o $100 o q Sabino 1 q Dun *AMHA membership applications can be found at www.morganhorse.com or by contacting AMHA. q Silver q Dominant White Pattern Reissue certificate with coat color test results .................................................. $25 o $110 o (original certificate must be submitted) q Lethal White Overo I understand that upon receipt of this application and the