Sequestered Standing Carbon Stock in Selective Exploited Timbers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Luthier's Dictionary
LUTHIER’S DICTIONARY ©2014 The European Guitar Builders Association ©2014 The European Guitar Builders Association Woods ENGLISH DUTCH GERMAN FINNISH FRENCH ITALIAN SPANISH SWEDISH LATIN Alder Elzen Erle Leppä Aulne Ontano Aliso Al Alnus Alder / european, black, Elzen / Europees, Erle Leppä / eurooppalainen Aulne européen Ontano europeo Aliso Europeo Al (Klibbal) Alnus glutinosa common zwart, gewoon Alder / red, western red Elzen Erle Leppä / amerikkalainen Aulne rouge, Aulne Ontano americano Aliso Rojo Rödal Alnus rubra américain Arctic Birch Berken Birke Jääkoivu Bouleau arctique Betulla artica (bianca) Abedul del Ártico Björk Betula pendula Ash Essen Esche Saarni Frêne Frassino Fresno Ask Fraxinus Ash / american Amerikaans essen Esche Saarni / amerikkalainen Frêne américain Frassino americano Fresno Americano Vitask Fraxinus americana Ash / European Europees essen Esche Saarni / eurooppalainen Frêne européen Frassino europeo Fesno europeo Europeisk ask Fraxinus excelsior Ash / Swamp Ash Moeras essen Sumpfesche Suosaarni Frêne des marais Frassino palustre Fesno del Pantano Svartask Fraxinus nigra Basswood Linden Linde Lehmus Tilleul Tiglio Tilo Lind Tilia americana Bubinga Bubinga Bubinga Bubinga Bubinga Bubinga Bubinga Bubinga Guibourtia spp. (G. demeusei, G. pellegriniana, G. tessmannii, etc.) Cocobolo Cocobolo Cocobolo Cocobolo Coccobolo Cocobolo Cocobolo Mexikansk jakaranda Dalbergia retusa Ebony / African Afrikaans ebben Ebenholz Eebenpuu / Ébène Ebano africano Ébano Africano Ebenholts Diospyros crassiflora afrikkalainen Ebony / -
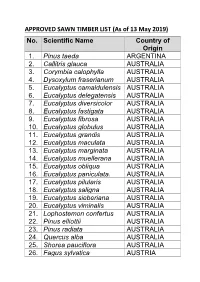
APPROVED SAWN TIMBER LIST (As of 13 May 2019) No. Scientific Name Country of Origin 1
APPROVED SAWN TIMBER LIST (As of 13 May 2019) No. Scientific Name Country of Origin 1. Pinus taeda ARGENTINA 2. Callitris glauca AUSTRALIA 3. Corymbia calophylla AUSTRALIA 4. Dysoxylum fraserianum AUSTRALIA 5. Eucalyptus camaldulensis AUSTRALIA 6. Eucalyptus delegatensis AUSTRALIA 7. Eucalyptus diversicolor AUSTRALIA 8. Eucalyptus fastigata AUSTRALIA 9. Eucalyptus fibrosa AUSTRALIA 10. Eucalyptus globulus AUSTRALIA 11. Eucalyptus grandis AUSTRALIA 12. Eucalyptus maculata AUSTRALIA 13. Eucalyptus marginata AUSTRALIA 14. Eucalyptus muellerana AUSTRALIA 15. Eucalyptus obliqua AUSTRALIA 16. Eucalyptus paniculata. AUSTRALIA 17. Eucalyptus pilularis AUSTRALIA 18. Eucalyptus saligna AUSTRALIA 19. Eucalyptus sieberiana AUSTRALIA 20. Eucalyptus viminalis AUSTRALIA 21. Lophostemon confertus AUSTRALIA 22. Pinus elliottii AUSTRALIA 23. Pinus radiata AUSTRALIA 24. Quercus alba AUSTRALIA 25. Shorea pauciflora AUSTRALIA 26. Fagus sylvatica AUSTRIA No. Scientific Name Country of Origin 27. Picea abies AUSTRIA 28. Picea abies BELARUS 29. Pinus sylvestris BELARUS 30. Quercus alba BELGIUM 31. Dipteryx odorata BOLIVIA 32. Apuleia leiocarpa BRAZIL 33. Astronium lecointei BRAZIL 34. Bagassa guianensis BRAZIL 35. Cedrela odorata BRAZIL 36. Cedrelinga catenaeformis BRAZIL 37. Couratari guianensis BRAZIL 38. Dipteryx odorata BRAZIL 39. Eucalyptus grandis BRAZIL 40. Eucalyptus grandis BRAZIL 41. Hymenaea courbaril BRAZIL 42. Hymenolobium modestum BRAZIL 43. Hymenolobium Nitidum BRAZIL Benth 44. Hymenolobium BRAZIL pulcherrimum 45. Manilkara bidentata BRAZIL 46. Myroxylon balsamum BRAZIL 47. Pinus radiata BRAZIL 48. Pinus taeda BRAZIL 49. Quassia simarouba BRAZIL 50. Tectona grandis BRAZIL 51. Fagus sylvatica BULGARIA No. Scientific Name Country of Origin 52. Quercus alba BULGARIA 53. Chlorophora excelsa CAMEROON 54. Cylicodiscus gabunensis CAMEROON 55. Entandrophragma CAMEROON candollei 56. Entandrophragma CAMEROON cylindricum 57. Entandrophragma CAMEROON cylindricum 58. Entandrophragma utile CAMEROON 59. -

Chapter 3--Physical Properties and Moisture Relations of Wood
Chapter 3 Physical Properties and Moisture Relations of Wood William Simpson and Anton TenWolde he versatility of wood is demonstrated by a wide Contents variety of products. This variety is a result of a Appearance 3–1 spectrum of desirable physical characteristics or properties among the many species of wood. In many cases, Grain and Texture 3–1 more than one property of wood is important to the end Plainsawn and Quartersawn 3–2 product. For example, to select a wood species for a product, the value of appearance-type properties, such as texture, grain Decorative Features 3–2 pattern, or color, may be evaluated against the influence of Moisture Content 3–5 characteristics such as machinability, dimensional stability, Green Wood and Fiber Saturation Point 3–5 or decay resistance. Equilibrium Moisture Content 3–5 Wood exchanges moisture with air; the amount and direction of the exchange (gain or loss) depend on the relative humid- Sorption Hysteresis 3–7 ity and temperature of the air and the current amount of water Shrinkage 3–7 in the wood. This moisture relationship has an important Transverse and Volumetric 3–7 influence on wood properties and performance. This chapter discusses the physical properties of most interest in the Longitudinal 3–8 design of wood products. Moisture–Shrinkage Relationship 3–8 Some physical properties discussed and tabulated are influ- Weight, Density, and Specific Gravity 3–11 enced by species as well as variables like moisture content; Working Qualities 3–15 other properties tend to be independent of species. The thor- oughness of sampling and the degree of variability influence Decay Resistance 3–15 the confidence with which species-dependent properties are Thermal Properties 3–15 known. -

FSC Public Search
CERTIFICATE Information from 2018/08/28 - 14:26 UTC Certificate Code CU-COC-816023 License Code FSC-C102167 MAIN ADDRESS Name Timber Link International Ltd. Address The Timber Office,Hazelwood Cottage,Maidstone Road,Hadlow Tonbridge TN11 0JH Kent UNITED KINGDOM Website http://www.timberlinkinternational.com CERTIFICATE DATA Status Valid First Issue Date 2010-10-16 Last Issue Date 2017-01-12 Expiry Date 2022-01-11 Standard FSC-STD-40-004 V3-0 GROUP MEMBER/SITES No group member/sites found. PRODUCTS Product Trade Species Primary Secondary Main Type Name Activity Activity Output Category W5 Solid Acer spp.; Alnus rubra var. pinnatisecta Starker; Alnus brokers/traders FSC wood serrulata; Apuleia leiocarpa; Betula spp.; Castanea sativa without physical Mix;FSC (sawn, P.Mill.; Cedrela odorata; Cedrus libani A. Rich.; Chlorocardium posession 100% chipped, rodiei (R.Schomb.) R.R.W.; Cylicodiscus gabunensis (Taub.) peeled) Harms; Dicorynia guianensis Amsh., D. paraensis Benth.; W5.2 Solid Dipterocarpus spp; Dipteryx odorata; Dryobalanops spp.; wood Dyera costulata (Miq.) Hook.f.; Entandrophragma cylindricum; boards Entandrophragma spp.; Entandrophragma utile; Eucalyptus spp; Fagus sylvatica L.; Fraxinus excelsior; Fraxinus americana; Gonystylus bancanus; Guibourtia spp.; Hymenaea courbaril; Intsia bijuga; Juglans nigra L.; Juglans regia L.; Khaya spp.; Larix sibirica; Liriodendron tulipifera L.; Lophira alata; Manilkara bidentata (A.DC.) A.Chev.; Microberlinia spp.; Milicia excelsa; Millettia laurentii; Nauclea diderrichii; Parashorea spp. (Urat mata, white seraya, gerutu); Peltogyne spp.*; Pinus rigida; Platanus occidentalis L; Prunus avium; Prunus serotina Ehrh.; Pseudotsuga menziesii; Pterocarpus soyauxii; Quercus alba; Quercus petraea; Quercus robur; Robinia pseudoacacia L.; Shorea balangeran; Shorea laevis Ridl.; Shorea spp.; Swietenia macrophylla; Tabebuia spp.; Tectona grandis; Terminalia ivorensis A. -

Wood Toxicity: Symptoms, Species, and Solutions by Andi Wolfe
Wood Toxicity: Symptoms, Species, and Solutions By Andi Wolfe Ohio State University, Department of Evolution, Ecology, and Organismal Biology Table 1. Woods known to have wood toxicity effects, arranged by trade name. Adapted from the Wood Database (http://www.wood-database.com). A good reference book about wood toxicity is “Woods Injurious to Human Health – A Manual” by Björn Hausen (1981) ISBN 3-11-008485-6. Table 1. Woods known to have wood toxicity effects, arranged by trade name. Adapted from references cited in article. Trade Name(s) Botanical name Family Distribution Reported Symptoms Affected Organs Fabaceae Central Africa, African Blackwood Dalbergia melanoxylon Irritant, Sensitizer Skin, Eyes, Lungs (Legume Family) Southern Africa Meliaceae Irritant, Sensitizer, African Mahogany Khaya anthotheca (Mahogany West Tropical Africa Nasopharyngeal Cancer Skin, Lungs Family) (rare) Meliaceae Irritant, Sensitizer, African Mahogany Khaya grandifoliola (Mahogany West Tropical Africa Nasopharyngeal Cancer Skin, Lungs Family) (rare) Meliaceae Irritant, Sensitizer, African Mahogany Khaya ivorensis (Mahogany West Tropical Africa Nasopharyngeal Cancer Skin, Lungs Family) (rare) Meliaceae Irritant, Sensitizer, African Mahogany Khaya senegalensis (Mahogany West Tropical Africa Nasopharyngeal Cancer Skin, Lungs Family) (rare) Fabaceae African Mesquite Prosopis africana Tropical Africa Irritant Skin (Legume Family) African Padauk, Fabaceae Central and Tropical Asthma, Irritant, Nausea, Pterocarpus soyauxii Skin, Eyes, Lungs Vermillion (Legume Family) -

Wood: Materials for Furniture 1
Wood: Materials for Furniture 1. Style and Design The style, or general appearance of furniture during a The furniture industry has relied heavily on wood and period of time, changes over the course of history. wood-based materials throughout history. Furniture Style reflects the priorities of the culture at the time. has evolved from simple utilitarian objects made to The furniture of early civilizations reflected the desire support people and inanimate objects to articles that of the privileged and ruling classes to display their are designed and built with both functional and power, wealth, or zealous aspirations. From the aesthetic characteristics and intended for display as beginning, furniture style was influenced by archi- well as utility. The origin and development of furniture tectural style. Ample evidence exists indicating that can be traced to an innate human desire to provide for Egyptian architectural ornamentation was applied to comfort and convenience as well as to display wealth, ancient Egyptian furniture and changes in Greek social and political status, or power. Furniture is made architecture were reflected in Greek furniture styles. from many materials, from rattan to precious metals, Beginning in the late nineteenth century, architects but from the earliest recorded history to the present, began having a direct influence in the design of the dominant material chosen for constructing fur- furniture, attributable to their desire to furnish niture is wood (see Wood: History of Use). Wood buildings in harmony with the overall design of a furniture was present in the ancient cultures of China, structure. The common element among all of these Egypt, and Greece. -

Basic Information on the Two Farms and Samartex Company
BASIC INFORMATION ON THE TWO FARMS AND SAMARTEX COMPANY NANA ESSANDOH’S FARM Nana Essandoh is a farmer with land size of 70 acres. He was the District Best Farmer in the year 2015. 50 acres of the land is cocoa intercropped with different Timber Tree species. 6 acres of the land is Teak Plantation whilst the remaining 14 acres is for other economic trees species. Examples of these Trees species are Milicia excels (Odum), Entandrophragma cylindricum (Sapele), Triplochiton scleroxylon (Wawa), Cedrela odorata (Cedrela), Khaya ivorensis (Mahogany), Terminalia superba (Ofram), Entandrophragma angolense (Edinam), among others. He has over 6000 tree species, 3000 of which are registered. He also, has 14 acres of Rubber plantation and substantial livestock. QUARM FARMS Mr. Sylvester Quarm is a renowned farmer in the landscape. The J. Y Quarm farms is well known in the country as a model agroforestry farm which has been a huge resource for research and practical demonstrations for students in the country’s tertiary institutions. Mr Quarm has won the Regional Best Farmer title for Western Region a number of times and winning the ultimate title of National Best Farmer is one of his ambitions. The farm is about 150 acres with mixed uses- timber tree plantations, cash crop plantations, food crops, livestock, etc. Some of the tree species including; Milicia excels (Odum), Entandrophragma cylindricum (Sapele), Triplochiton scleroxylon (Wawa), Cedrela odorata (Cedrela), Khaya ivorensis (Mahogany), Terminalia superba (Ofram), Entandrophragma angolense (Edinam), among others. The farm also has a rubber plantation, cocoa under shade trees, teak plantation, oranges, coconut, and food crops such as plantain, cassava, etc. -

Entandrophragma Cylindricum Family: Meliaceae Sapele
Entandrophragma cylindricum Family: Meliaceae Sapele Other Common Names: Aboudikro (Ivory Coast), Penkwa (Ghana), Muyovu (Uganda), Sapelii (Cameroon), Libuyu (Zaire). Distribution: Ranging from the Ivory Coast to the Cameroons and eastward through Zaire to Uganda. Occurs in evergreen, deciduous, and transitional forest formation. The Tree: May reach a height of 150 to 200 ft; bole straight and cylindrical, clear to 100 ft; trunk diameters to 6 ft over broad, low buttresses, sometimes not buttressed. The Wood: General Characteristics: Heartwood a medium to fairly dark reddish brown or purplish brown; sapwood whitish or pale yellow, distinct. Texture rather fine; grain interlocked, sometimes wavy, producing a narrow, uniform, roe figure on quartered surfaces; lustrous; without a distinctive taste but with a cedarlike scent. Weight: Basic specific gravity (ovendry weight/green volume) about 0.55; air-dry density 42 pcf. Mechanical Properties: (2-cm standard) Moisture content Bending strength Modulus of elasticity Maximum crushing strength (%) (Psi) (1,000 psi) (Psi) Green (9) 10,700 1,390 5,220 12% 16,100 1,700 5,500 12% (26) 16,500 1,700 8,900 Janka side hardness 1,020 lb for green and 1,500 lb for dry material. Amsler toughness 200 in.-lb for dry material (2-cm specimen). Drying and Shrinkage: Seasons fairly rapidly but with a marked tendency to warp, very variable in drying properties, requires careful stacking. Kiln schedule T2- D4 is suggested for 4/4 stock and T2- D3 for 8/4. Shrinkage green to ovendry: radial 4.6%; tangential 7.4%; volumetric 14.0%. Movement in service is rated as medium. -

An Updated List of Species Used in Tree-Ring Research
TREE-RING BULLETIN, Vol. 53, 1993 AN UPDATED LIST OF SPECIES USED IN TREE-RING RESEARCH HENRI D. GRISSINO-MAYER Laboratory of Tree-Ring Research University of Arizona Tucson, AZ 85721, U.S.A. ABSTRACT During the past 100 years, researchers have investigated the potential of hundreds of tree and shrub species for use in applications of tree-ring research. Although several lists of species known to crossdate have been published, investigated species that do not crossdate are rarely included despite the usefulness of this infonnation for future research. This paper provides a list of the Latin and common names of 573 species that have been investigated in tree-ring research, infor mation on species known to crossdate, and information on species with measurement and/or chronology data in the International Tree-Ring Data Bank. In addition, a measure of the suitability of a species for future tree-ring applications, the Crossdating Index (CDI), is developed and pro posed for standard usage. 1n den letzten hundert J ahren haben Forscher das Potential von hunderten von Baum- und Buscharten fi.ir die Anwendung in der Jahresring-Forschung untersucht. Zahlreiche Listen mit Arten, von denen man wei~, da~ sie zeitlich korrespondieren, sind bereits veroffentlicht worden, dagegen sind untersuchte Arten, die nicht zeitlich korresponclieren, selten in Publikationen beriick sichtigt worden, obwohl diese Informationen fi.ir die kiinftige Forschung nutzvoll sein konnten. Dieser Artikel legt eine Liste der lateinischen und der gemeinen Narnen von 573 Arten vor, die im Rahmen der Jahresring-Forschung untersucht worden sind, Inforrnationen Uber Arten, die bekan nterweise zeitlich korrespondieren sowie Informationen iiber Arten mit Ma~- und/oder Chronologiedaten in der intemationalen Jahresring-Datenbank (International Tree-Ring Data Bank). -

Entandrophragma Cylindricum Meliaceae
Entandrophragma cylindricum Meliaceae Indigenous Trade names: Sapele, muyovu. Common names: Luganda: Muyovu Runyoro: Muyovu Rutoro: Muyovu. Ecology: Sepele is the name of a Nigerian town. This tree is an important timber tree occurring widely in tropical Africa from Sierra Leone to Uganda and Zaire. In Uganda is grows in mixed to climax tropical rain forest, in thickets and in gallery forest in Budongo, Bugoma, Mabira and West Mengo Forests, 1,100-1,500 m. Uses: Firewood, charcoal, timber (furniture), veneer, shade, ornamental (avenues). Description: A deciduous forest tree to 55 m or more, the trunk tall and straight, often clear 25-30 m, the rounded crown medium sized. Buttresses alone up to 3 m. The trunk may be 1 m or more across. BARK: brown and smooth at first, turning grey and flaking towards the base in irregular scales on mature trees. LEAVES: pinnate on stalks to 30 cm, tufted at the ends of branches, 11-19 leaflets, often alternate, lowest pairs oval, others long oval up to 12 cm long, tip pointed, 6-9 lateral veins and a close network of veins on both surfaces. FLOWERS: tiny, white on a branched stalk to 25 cm. FRUIT: a brown woody capsule about 14 cm, rounded at the tip, breaking into 5 parts. The capsule opens first at the tip, then the base and pieces fall away one at a time. Winged seeds about 8 cm long are attached alternately left and right to the central column. Propagation: Seedlings, wildings. Seed: The winged seeds get blown several metres away from the mother tree. -

Thermochemical Properties of Several Portuguese and Exotic Woods And
José Luis LOUSADA1 Thermochemical Maria Emília SILVA1 properties of several Rafael SCHMITZ2 1Center for the Reserch and Technology of Agro-Environmental and Biological Sciences (CITAB) / UTAD – Vila Real, Portugal Portuguese and exotic 2Instituto Federal de Santa Catarina IF-SC, São Miguel do Oeste, Santa Catarina, Brasil. woods and shrubs 1 Background In recent years it has been attended in Portugal a constant increase in demand for forest biomass for energy purposes, which may lead to an overexploitation of these resources. Only an efficient management of biomass can ensure the sustainability of the Portuguese Forest. This can be achieved by the use of biomass resources yet unexplored (shrubs) or by producing more biomass (energy crops). The aim of this study was to evaluate the thermochemical properties of various national wood species, exotic species, and shrubs, most representative of Portugal . 2 Methods The study assessed in national softwoods, national and tropical hardwoods and shrub species. For each species it was determined the gross calorific value-GCV (or higher heating value-HHV) and the following chemical properties: ash content, chemical composition (C, H, O, N, S), Micro and Macroelements (Na, K, Ca, Mg, Mn, Fe, Zn, Ni, Cr, Cd, Cu, P) and halogens (F, Br, Cl). 3 Results Ulex europaeus Ulex europaeus Ulex europaeus Tojo Tojo Ulex europaeus Cytisus striatus Cytisus striatus Cytisus striatusTojo Tojo GROSS CALORIFIC VALUE ASH CONTENT Giesta Giesta Giesta Cytisus striatusGiesta Hakea sericea Hakea Hsericeaaquea Hakea Hsericeaaquea Hakea Hsericeaaquea Haquea Erica sp EricaErica s spp. Erica sp. EEricarica s spp. EEricarica s spp. Erica arborea Erica arborea Erica arborea Erica arborea EricaErica aarborearborea EricaErica aarborearborea PterospartumCarq utrid.eja PterospartumCarqu trid.eja PterospartumCarq utrid.eja PterospartumCarq utrid.eja Hymenaea Softwoods GCV (kJ/kg) Softwoods Ash(%) HHymenaeaymenaea c. -

Certified Wood Species*
M. Bohlke Corp. 8375 N. Gilmore Road Fairfield, OH P: (513) 874-4400 mbveneer.com [email protected] Wood is Green Preserving and enhancing our natural resources. We have a strong commitment to the preservation and protection of the forests that supply our raw materials. We comply with all forestry standards and regulations by working with timberland owners to responsibly harvest their trees in a selective manner. By purchasing our trees and logs from private landowners and government-owned properties that hire state-certified foresters to mark mature trees for harvest, we are preserving our forests by leaving the smaller trees for further growth and future generations. Through selective harvesting, America’s hardwood forests have been able to regenerate more trees than are harvested. Most trees we use today come from third- or fourth-cut hardwood stands, allowing the temperate hardwoods of North America to regenerate to full mature age within one human lifetime. Only mature trees at the end of their life cycle are viable for veneer manufacturing – young trees do not yield a fine veneer product. For every one hundred trees in the forest, only about seven of them are suitable for veneer quality. In a time when timber regulations are becoming much more stringent, we pride ourselves on carrying a solid inventory of certified veneer and lumber. Responsible utilization of timber mandates that we be productive in our manufacturing processes to produce high yields from our raw material. For years, M. Bohlke Corp. has invested, developed and patented the latest technology to produce the most out of logs.