Amphibian Conservation Resource Manual
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CAT Vertebradosgt CDC CECON USAC 2019
Catálogo de Autoridades Taxonómicas de vertebrados de Guatemala CDC-CECON-USAC 2019 Centro de Datos para la Conservación (CDC) Centro de Estudios Conservacionistas (Cecon) Facultad de Ciencias Químicas y Farmacia Universidad de San Carlos de Guatemala Este documento fue elaborado por el Centro de Datos para la Conservación (CDC) del Centro de Estudios Conservacionistas (Cecon) de la Facultad de Ciencias Químicas y Farmacia de la Universidad de San Carlos de Guatemala. Guatemala, 2019 Textos y edición: Manolo J. García. Zoólogo CDC Primera edición, 2019 Centro de Estudios Conservacionistas (Cecon) de la Facultad de Ciencias Químicas y Farmacia de la Universidad de San Carlos de Guatemala ISBN: 978-9929-570-19-1 Cita sugerida: Centro de Estudios Conservacionistas [Cecon]. (2019). Catálogo de autoridades taxonómicas de vertebrados de Guatemala (Documento técnico). Guatemala: Centro de Datos para la Conservación [CDC], Centro de Estudios Conservacionistas [Cecon], Facultad de Ciencias Químicas y Farmacia, Universidad de San Carlos de Guatemala [Usac]. Índice 1. Presentación ............................................................................................ 4 2. Directrices generales para uso del CAT .............................................. 5 2.1 El grupo objetivo ..................................................................... 5 2.2 Categorías taxonómicas ......................................................... 5 2.3 Nombre de autoridades .......................................................... 5 2.4 Estatus taxonómico -

Amazophrynella Minuta (Amphibia: Anura: Bufonidae) Da Região Amazônica
UNIVERSIDADE FEDERAL DO AMAZONAS-UFAM INSTITUTO DE CIÊNCIAS BIOLÓGICAS-ICB PROGRAMA DE PÓS GRADUAÇÃO EM DIVERSIDADE BIOLÓGICA - PPGDIVBIO Revisão taxonômica e distribuição geográfica do complexo Amazophrynella minuta (Amphibia: Anura: Bufonidae) da região Amazônica ROMMEL ROBERTO ROJAS ZAMORA Manaus- Amazonas Março - 2014 1 UNIVERSIDADE FEDERAL DO AMAZONAS-UFAM INSTITUTO DE CIÊNCIAS BIOLÓGICAS-ICB PROGRAMA DE PÓS GRADUAÇÃO EM DIVERSIDADE BIOLÓGICA - PPGDIVBIO Revisão taxonômica e distribuição geográfica do complexo Amazophrynella minuta (Amphibia: Anura: Bufonidae) da região Amazônica ROMMEL ROBERTO ROJAS ZAMORA Orientador: Prof. Dr. TOMAS HRBEK Co-Orientador: Prof. Dr. MARCELO GORDO Dissertação apresentada à . Coordenação do Programa de Pós-Graduação em Diversidade Biológica, Universidade Federal do Amazonas, como requisito parcial para obtenção do título de mestre em Diversidade Biológica Manaus - Amazonas Março - 2014 2 Ficha Catalográfica (Catalogação realizada pela Biblioteca Central da UFAM) Rojas Zamora, Rommel Roberto Revisão taxonômica e distribuição geográfica do complexo R741r Amazophrynella minuta (Amphibia: Anura: Bufonidae) da região Amazônica / Rommel Roberto Rojas Zamora. - 2014. 83 f. : il. color. ; 31 cm. Dissertação (mestre em Diversidade Biológica) –– Universidade Federal do Amazonas. Orientador: Prof. Dr. Tomas Hrbek. Co-orientador: Prof. Dr. Marcelo Gordo. 1. Anuro – Classificação - Amazônia 2. Anfíbio – Classificação – Amazônia 3. Bufo – Classificação – Amazônia 4. Anuro – Criação 5. Anfíbio – Criação 6. Bufo – Criação I. Hrbek, Tomas, orientador II. Gordo, Marcelo, orientador III. Universidade Federal do Amazonas IV. Título CDU (2007): 597.8(811)(043.3) iii 3 Poucas vezes são as ocasiões onde um filho tem a oportunidade de agradecer o esforço do seus pais; esta é uma de elas. Dedico esta teses aos meus queridos Pais (Elvira e Roberto) por cuidar com empenho sua prole. -

Eugenio Izecksohn 1 O Gênero Dendrophryniscus Jiménez De La
TR~S NOVAS ESPÉCIES DE DENDROPHRYNISCUS JIMÉNEZ DE LA ESPADA DAS REGiÕES SUDESTE E SUL DO BRASIL (AMPHIBIA, ANURA, BUFONIDAE) Eugenio Izecksohn 1 ABSTRACT. THREE NEW SPECIES OF DENDROPHRYNISCUS J1MÉNEZ DE LA ESPADA FROM SOUTHEAST AND SOUTH REGIONS OF BRAZIL (AMPHIBIA, ANURA, BUFONIDAE). Dendrophryniscus carvalhoi, sp.n. from Espírito Santo, D. berthalutzae, sp.n. from Santa Catarina, and D. slawiarskyi, sp.n. from Paraná, Brazil, are described and considered to be related to D. brevipollicatus Jiménez de la Espada. KEY WORDS. Amphibia, Anura, Bufonidae, taxonomy of frogs o gênero Dendrophryniscus Jiménez de la Espada, reúne pequenos anuros de corpo relativamente alongado, com colorido pardacento, que pa recem habitar exclusivamente florestas. Menos vistosos que a espécies dos gêneros afins Ate/opus Duméril & Bibron e Me/anophryniscus Gallardo, muito homogêneos em seu aspecto, com voz débil e quase sempre ocultos em bainhas de folhas ou disfarçados no chão das matas, talvez por isso tenham permanecido pouco estudados. A descontinuidade na distribuição do gênero é acentuada, sendo conhe cidos alguns representantes nas florestas do sudeste e sul brasileiros e outros na Bacia Amazônica, no Brasil, Guiana, Colômbia e Equador. Dendrophryniscus brevipollicatus Jiménez de la Espada, do sudeste bra sileiro, como deve ocorrer com algumas formas afins, tem ontogênese es pecializada, estando suas larvas adaptadas a vida em bromeliáceas (LUTZ, 1932; CARVALHO, 1949; IZECKSOHN & CRUZ, 1972); D. leucomystax Izecksohn, D. minutus Melin e provavelmente D. bokemtanni Izecksohn criam suas larvas em poças de água acumuladas no solo (DUELLMAN & L YNCH, 1969; IZECKSOHN & CRUZ, 1972; IZECKSOHN, no prelo). No presente trabalho são feitas considerações sobre D. -

Effects of Emerging Infectious Diseases on Amphibians: a Review of Experimental Studies
diversity Review Effects of Emerging Infectious Diseases on Amphibians: A Review of Experimental Studies Andrew R. Blaustein 1,*, Jenny Urbina 2 ID , Paul W. Snyder 1, Emily Reynolds 2 ID , Trang Dang 1 ID , Jason T. Hoverman 3 ID , Barbara Han 4 ID , Deanna H. Olson 5 ID , Catherine Searle 6 ID and Natalie M. Hambalek 1 1 Department of Integrative Biology, Oregon State University, Corvallis, OR 97331, USA; [email protected] (P.W.S.); [email protected] (T.D.); [email protected] (N.M.H.) 2 Environmental Sciences Graduate Program, Oregon State University, Corvallis, OR 97331, USA; [email protected] (J.U.); [email protected] (E.R.) 3 Department of Forestry and Natural Resources, Purdue University, West Lafayette, IN 47907, USA; [email protected] 4 Cary Institute of Ecosystem Studies, Millbrook, New York, NY 12545, USA; [email protected] 5 US Forest Service, Pacific Northwest Research Station, Corvallis, OR 97331, USA; [email protected] 6 Department of Biological Sciences, Purdue University, West Lafayette, IN 47907, USA; [email protected] * Correspondence [email protected]; Tel.: +1-541-737-5356 Received: 25 May 2018; Accepted: 27 July 2018; Published: 4 August 2018 Abstract: Numerous factors are contributing to the loss of biodiversity. These include complex effects of multiple abiotic and biotic stressors that may drive population losses. These losses are especially illustrated by amphibians, whose populations are declining worldwide. The causes of amphibian population declines are multifaceted and context-dependent. One major factor affecting amphibian populations is emerging infectious disease. Several pathogens and their associated diseases are especially significant contributors to amphibian population declines. -

Masked Bobwhite (Colinus Virginianus Ridgwayi) 5-Year Review
Masked Bobwhite (Colinus virginianus ridgwayi) 5-Year Review: Summary and Evaluation Photograph by Paul Zimmerman U.S. Fish and Wildlife Service Buenos Aires National Wildlife Refuge Sasabe, AZ March 2014 5-YEAR REVIEW Masked Bobwhite (Colinus virginianus ridgwayi) 1.0 GENERAL INFORMATION 1.1 Reviewers Lead Regional Office Southwest Region, Region 2, Albuquerque, NM Susan Jacobsen, Chief, Division of Classification and Restoration, 505-248-6641 Wendy Brown, Chief, Branch of Recovery and Restoration, 505-248-6664 Jennifer Smith-Castro, Recovery Biologist, 505-248-6663 Lead Field Office: Buenos Aires National Wildlife Refuge (BANWR) Sally Gall, Refuge Manager, 520-823-4251 x 102 Juliette Fernandez, Assistant Refuge Manager, 520-823-4251 x 103 Dan Cohan, Wildlife Biologist, 520-823-4351 x 105 Mary Hunnicutt, Wildlife Biologist, 520-823-4251 Cooperating Field Office(s): Arizona Ecological Services Tucson Field Office Jean Calhoun, Assistant Field Supervisor, 520-670-6150 x 223 Mima Falk, Senior Listing Biologist, 520-670-6150 x 225 Scott Richardson, Supervisory Fish and Wildlife Biologist, 520-670-6150 x 242 Mark Crites, Fish and Wildlife Biologist, 520-670-6150 x 229 Arizona Ecological Services Field Office Steve Spangle, Field Supervisor, 602-242-0210 x 244 1.2 Purpose of 5-Year Reviews: The U.S. Fish and Wildlife Service (Service or USFWS) is required by section 4(c)(2) of the Endangered Species Act (Act) to conduct a status review of each listed species once every 5 years. The purpose of a 5-year review is to evaluate whether or not the species’ status has changed since it was listed (or since the most recent 5-year review). -

FEB Craig Guyer - 4 1999 Department of Zoology and Wildlife Science Auburn University Auburn, AL 36849 (334)-844-9232 [email protected]
HISTORICAL AFFINITIES AND POPULATION BIOLOGY OF THE BLACK WARRIOR WATERDOG (NECTURUS ALABAMENSIS) FINAL REPORT FY 1998 FEB - 4 1999 Craig Guyer Department of Zoology and Wildlife Science Auburn University Auburn, AL 36849 (334)-844-9232 [email protected] SUMMARY 1) The Black Warrior waterdog is morphologically and genetically distinctive from other waterdogs in the state of Alabama and should be recognized as Necturus alabamensis. 2) The Black Warrior waterdog is most closely related to the mudpuppy, Necturus maculosus. 3) Four waterdogs are present in the state of Alabama, the two listed above, plus two forms from the Coastal Plains; the latter include Necturus beyeri (all rivers draining into Mobile Bay) and Necturus iodingi (Appalachicola to Perdido drainages, inclusive). 4) Populations of Black Warrior waterdogs in Sipsey Fork and Brushy Creek appear to be patchily distributed, creating challenges for determining key features of demography. 5) State and Federal protection of the Black Warrior waterdog as a threatened species is warranted. INTRODUCTION Waterdogs (Necturus: Proteidae) are paedomorphic, stream-dwelling salamanders of the Atlantic and Gulf Coastal Plains. The systematics of these creatures has challenged herpetologists for the past 60 years. The Black Warrior Waterdog, a species restricted to the upper Black Warrior drainages of Alabama, has been particularly problematic. Viosca (1937) originally described this taxon as being similar toN maculosus, but subsequent taxonomic treattnents considered specimens from this drainage to be conspecific with waterdogs from the lower portions of the Mobile drainages (N maculosus: Bishop 1943, Schmidt 1953; N beyeri alabamensis: Hecht 1958, Conant 1958; N puncta/us: Brode 1969; N beyeri: Mount 1975; N alabamensis: Conant 1975, Conant and Collins 1998). -

Pre-Incursion Plan PIP006 Salamanders and Newts
Pre-incursion Plan PIP006 Salamanders and Newts Pre-incursion Plan PIP006 Salamanders and Newts Order: Ambystomatidae, Cryptobranchidea and Proteidae Scope This plan is in place to guide prevention and eradication activities and the management of non-indigenous populations of Salamanders and Newts (Order Caudata; Families Salamandridae, Ambystomatidae, Cryptobranchidea and Proteidae) amphibians in the wild in Victoria. Version Document Status Date Author Reviewed By Approved for Release 1.0 First Draft 26/07/11 Dana Price M. Corry, S. Wisniewski and A. Woolnough 1.1 Second Draft 21/10/11 Dana Price S. Wisniewski 2.0 Final Draft 18/01/2012 Dana Price 3.0 Revision Draft 12/11/15 Dana Price J. Goldsworthy 3.1 New Final 10/03/2016 Nigel Roberts D.Price New DEDJTR templates and document review Published by the Department of Economic Development, Jobs, Transport and Resources, Agriculture Victoria, May 2016 © The State of Victoria 2016. This publication is copyright. No part may be reproduced by any process except in accordance with the provisions of the Copyright Act 1968. Authorised by the Department of Economic Development, Jobs, Transport and Resources, 1 Spring Street, Melbourne 3000. Front cover: Smooth Newt (Lissotriton vulgaris) Photo: Image courtesy of High Risk Invasive Animals group, DEDJTR Photo: Image from Wikimedia Commons and reproduced with permission under the terms of the Creative Commons Attribution-Share Alike 2.5 Generic License. ISBN 078-1-925532-40-1 (pdf/online) Disclaimer This publication may be of assistance to you but the State of Victoria and its employees do not guarantee that the publication is without flaw of any kind or is wholly appropriate for your particular purposes and therefore disclaims all liability for any error, loss or other consequence which may arise from you relying on any information in this publication. -

Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca
Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca John F. Lamoreux, Meghan W. McKnight, and Rodolfo Cabrera Hernandez Occasional Paper of the IUCN Species Survival Commission No. 53 Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca John F. Lamoreux, Meghan W. McKnight, and Rodolfo Cabrera Hernandez Occasional Paper of the IUCN Species Survival Commission No. 53 The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily reflect those of IUCN or other participating organizations. Published by: IUCN, Gland, Switzerland Copyright: © 2015 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Citation: Lamoreux, J. F., McKnight, M. W., and R. Cabrera Hernandez (2015). Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca. Gland, Switzerland: IUCN. xxiv + 320pp. ISBN: 978-2-8317-1717-3 DOI: 10.2305/IUCN.CH.2015.SSC-OP.53.en Cover photographs: Totontepec landscape; new Plectrohyla species, Ixalotriton niger, Concepción Pápalo, Thorius minutissimus, Craugastor pozo (panels, left to right) Back cover photograph: Collecting in Chamula, Chiapas Photo credits: The cover photographs were taken by the authors under grant agreements with the two main project funders: NGS and CEPF. -

Vocalizations of Eight Species of Atelopus (Anura: Bufonidae) with Comments on Communication in the Genus
Copeia, 1990(3), pp. 631-643 Vocalizations of Eight Species of Atelopus (Anura: Bufonidae) With Comments on Communication in the Genus REGINALD B. COCROFT, ROY W. MCDIARMID, ALAN P. JASLOW AND PEDRO M. RUIZ-CARRANZA Vocalizations of frogs of the genus Atelopus include three discrete types of signals: pulsed calls, pure tone calls, and short calls. Repertoire composition is conservative across species. Repertoires of most species whose calls have been recorded contain two or three of these identifiable call types. Within a call type, details of call structure are very similar across species. This apparent lack of divergence in calls may be related to the rarity of sympatry among species of Atelopus and to the relative importance of visual communication in their social interactions. COMMUNICATION in frogs of the genus lopus by comparing the structure and behavioral Atelopus has been httle studied despite sev- context, where available, of calls of these species eral unique features of their biology that make with calls of other species reported in the lit- them an important comparative system in light erature. of the extensive work on other species of an- METHODS urans (Littlejohn, 1977; Wells, 1977; Gerhardt, 1988). First, the auditory system oí Atelopus is Calls were analyzed using a Kay Digital Sona- highly modified, and most species lack external Graph 7800, a Data 6000 Waveform Analyzer and middle ears (McDiarmid, 1971). Second, in with a model 610 plug-in digitizer, and a Mul- contrast to most anurans, species oiAtelopus are tigon Uniscan II real-time analyzer. Call fre- diurnal and often brightly colored, and visual quencies were measured from waveform and communication plays an important role in the Fourier transform analyses; pulse rates and call social behavior of at least some species (Jaslow, lengths were measured from waveform analyses 1979; Crump, 1988). -

DNR Letterhead
ATU F N RA O L T R N E E S M O T U STATE OF MICHIGAN R R C A P DNR E E S D MI N DEPARTMENT OF NATURAL RESOURCES CHIG A JENNIFER M. GRANHOLM LANSING REBECCA A. HUMPHRIES GOVERNOR DIRECTOR Michigan Frog and Toad Survey 2009 Data Summary There were 759 unique sites surveyed in Zone 1, 218 in Zone 2, 20 in Zone 3, and 100 in Zone 4, for a total of 1097 sites statewide. This is a slight decrease from the number of sites statewide surveyed last year. Zone 3 (the eastern half of the Upper Peninsula) is significantly declining in routes. Recruiting in that area has become necessary. A few of the species (i.e. Fowler’s toad, Blanchard’s cricket frog, and mink frog) have ranges that include only a portion of the state. As was done in previous years, only data from those sites within the native range of those species were used in analyses. A calling index of abundance of 0, 1, 2, or 3 (less abundant to more abundant) is assigned for each species at each site. Calling indices were averaged for a particular species for each zone (Tables 1-4). This will vary widely and cannot be considered a good estimate of abundance. Calling varies greatly with weather conditions. Calling indices will also vary between observers. Results from the evaluation of methods and data quality showed that volunteers were very reliable in their abilities to identify species by their calls, but there was variability in abundance estimation (Genet and Sargent 2003). -
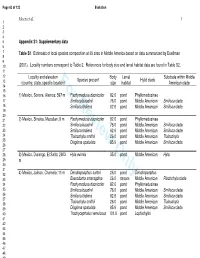
For Review Only
Page 63 of 123 Evolution Moen et al. 1 1 2 3 4 5 Appendix S1: Supplementary data 6 7 Table S1 . Estimates of local species composition at 39 sites in Middle America based on data summarized by Duellman 8 9 10 (2001). Locality numbers correspond to Table 2. References for body size and larval habitat data are found in Table S2. 11 12 Locality and elevation Body Larval Subclade within Middle Species present Hylid clade 13 (country, state, specific location)For Reviewsize Only habitat American clade 14 15 16 1) Mexico, Sonora, Alamos; 597 m Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 17 Smilisca baudinii 76.0 pond Middle American Smilisca clade 18 Smilisca fodiens 62.6 pond Middle American Smilisca clade 19 20 21 2) Mexico, Sinaloa, Mazatlan; 9 m Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 22 Smilisca baudinii 76.0 pond Middle American Smilisca clade 23 Smilisca fodiens 62.6 pond Middle American Smilisca clade 24 Tlalocohyla smithii 26.0 pond Middle American Tlalocohyla 25 Diaglena spatulata 85.9 pond Middle American Smilisca clade 26 27 28 3) Mexico, Durango, El Salto; 2603 Hyla eximia 35.0 pond Middle American Hyla 29 m 30 31 32 4) Mexico, Jalisco, Chamela; 11 m Dendropsophus sartori 26.0 pond Dendropsophus 33 Exerodonta smaragdina 26.0 stream Middle American Plectrohyla clade 34 Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 35 Smilisca baudinii 76.0 pond Middle American Smilisca clade 36 Smilisca fodiens 62.6 pond Middle American Smilisca clade 37 38 Tlalocohyla smithii 26.0 pond Middle American Tlalocohyla 39 Diaglena spatulata 85.9 pond Middle American Smilisca clade 40 Trachycephalus venulosus 101.0 pond Lophiohylini 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 Evolution Page 64 of 123 Moen et al. -

2006 Annual Report
2006 Annual Report Transforming passionate commitment to wildlife into effective conservation CONTENTS From the Executive Director 2 From the Chairman 3 About CBSG 4 2006 PHVA and CAMP Workshops / Sponsors 6 2006 Conservation Planning and Training Workshops / Sponsors 9 Success Stories: Saving Japan’s Tsushima Leopard Cat 10 Borderless Conservation for Bearded Vultures 11 Beach Mice: Living in the Eye of the Hurricane 12 Preserving Cuban Parrots 13 Returning Mexican Wolves to the Sierra Madre 14 Effecting Positive Change for Zoos and Animals 15 Special Report: Launching the Amphibian Ark 16 Core Team: CBSG Staff & Strategic Associates 18 CBSG Regional Networks 19 CBSG Conservation Council 20 CBSG Steering Committee 21 Financial Information 23 2006 Sponsors of CBSG Participation in Conservation Workshops and Meetings 24 2006 Ulysses S. Seal Award 24 OUR MISSION CBSG’s mission is to save threatened species by increasing the effectiveness of conservation efforts worldwide. Through: • innovative and interdisciplinary methodologies, • culturally sensitive and respectful facilitation, and • empowering global partnerships and collaborations, CBSG transforms passionate commitment to wildlife into effective conservation. CONSERVATION BREEDING SPECIALIST GROUP MEASURES OF SUCCESS In recent years, evaluation has been a prevalent issue in conservation conferences and the focus of discussion within the international zoo community. It has been a topic at CBSG Annual Meetings and is a key criterion in the development of recommendations in CBSG workshops. So naturally, when reflecting on the past year, I began thinking in terms of evaluation. There are some standard parameters we can use to evaluate CBSG as an organization, including top-line parameters such as organizational longevity, staff retention, and financial status.