ZM82-01 Nieukerken.Indd
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gori River Basin Substate BSAP
A BIODIVERSITY LOG AND STRATEGY INPUT DOCUMENT FOR THE GORI RIVER BASIN WESTERN HIMALAYA ECOREGION DISTRICT PITHORAGARH, UTTARANCHAL A SUB-STATE PROCESS UNDER THE NATIONAL BIODIVERSITY STRATEGY AND ACTION PLAN INDIA BY FOUNDATION FOR ECOLOGICAL SECURITY MUNSIARI, DISTRICT PITHORAGARH, UTTARANCHAL 2003 SUBMITTED TO THE MINISTRY OF ENVIRONMENT AND FORESTS GOVERNMENT OF INDIA NEW DELHI CONTENTS FOREWORD ............................................................................................................ 4 The authoring institution. ........................................................................................................... 4 The scope. .................................................................................................................................. 5 A DESCRIPTION OF THE AREA ............................................................................... 9 The landscape............................................................................................................................. 9 The People ............................................................................................................................... 10 THE BIODIVERSITY OF THE GORI RIVER BASIN. ................................................ 15 A brief description of the biodiversity values. ......................................................................... 15 Habitat and community representation in flora. .......................................................................... 15 Species richness and life-form -

National List of Vascular Plant Species That Occur in Wetlands 1996
National List of Vascular Plant Species that Occur in Wetlands: 1996 National Summary Indicator by Region and Subregion Scientific Name/ North North Central South Inter- National Subregion Northeast Southeast Central Plains Plains Plains Southwest mountain Northwest California Alaska Caribbean Hawaii Indicator Range Abies amabilis (Dougl. ex Loud.) Dougl. ex Forbes FACU FACU UPL UPL,FACU Abies balsamea (L.) P. Mill. FAC FACW FAC,FACW Abies concolor (Gord. & Glend.) Lindl. ex Hildebr. NI NI NI NI NI UPL UPL Abies fraseri (Pursh) Poir. FACU FACU FACU Abies grandis (Dougl. ex D. Don) Lindl. FACU-* NI FACU-* Abies lasiocarpa (Hook.) Nutt. NI NI FACU+ FACU- FACU FAC UPL UPL,FAC Abies magnifica A. Murr. NI UPL NI FACU UPL,FACU Abildgaardia ovata (Burm. f.) Kral FACW+ FAC+ FAC+,FACW+ Abutilon theophrasti Medik. UPL FACU- FACU- UPL UPL UPL UPL UPL NI NI UPL,FACU- Acacia choriophylla Benth. FAC* FAC* Acacia farnesiana (L.) Willd. FACU NI NI* NI NI FACU Acacia greggii Gray UPL UPL FACU FACU UPL,FACU Acacia macracantha Humb. & Bonpl. ex Willd. NI FAC FAC Acacia minuta ssp. minuta (M.E. Jones) Beauchamp FACU FACU Acaena exigua Gray OBL OBL Acalypha bisetosa Bertol. ex Spreng. FACW FACW Acalypha virginica L. FACU- FACU- FAC- FACU- FACU- FACU* FACU-,FAC- Acalypha virginica var. rhomboidea (Raf.) Cooperrider FACU- FAC- FACU FACU- FACU- FACU* FACU-,FAC- Acanthocereus tetragonus (L.) Humm. FAC* NI NI FAC* Acanthomintha ilicifolia (Gray) Gray FAC* FAC* Acanthus ebracteatus Vahl OBL OBL Acer circinatum Pursh FAC- FAC NI FAC-,FAC Acer glabrum Torr. FAC FAC FAC FACU FACU* FAC FACU FACU*,FAC Acer grandidentatum Nutt. -

Moths of Poole Harbour Species List
Moths of Poole Harbour is a project of Birds of Poole Harbour Moths of Poole Harbour Species List Birds of Poole Harbour & Moths of Poole Harbour recording area The Moths of Poole Harbour Project The ‘Moths of Poole Harbour’ project (MoPH) was established in 2017 to gain knowledge of moth species occurring in Poole Harbour, Dorset, their distribution, abundance and to some extent, their habitat requirements. The study area uses the same boundaries as the Birds of Poole Harbour (BoPH) project. Abigail Gibbs and Chris Thain, previous Wardens on Brownsea Island for Dorset Wildlife Trust (DWT), were invited by BoPH to undertake a study of moths in the Poole Harbour recording area. This is an area of some 175 square kilometres stretching from Corfe Castle in the south to Canford Heath in the north of the conurbation and west as far as Wareham. 4 moth traps were purchased for the project; 3 Mercury Vapour (MV) Robinson traps with 50m extension cables and one Actinic, Ultra-violet (UV) portable Heath trap running from a rechargeable battery. This was the capability that was deployed on most of the ensuing 327 nights of trapping. Locations were selected using a number of criteria: Habitat, accessibility, existing knowledge (previously well-recorded sites were generally not included), potential for repeat visits, site security and potential for public engagement. Field work commenced from late July 2017 and continued until October. Generally, in the years 2018 – 2020 trapping field work began in March/ April and ran on until late October or early November, stopping at the first frost. -

Leafminers Nunn & Warrington
More dots on the map: further records of leafmining moths in East Yorkshire Andy D. Nunn1 and Barry Warrington2 1Hull International Fisheries Institute, School of Biological, Biomedical & Environmental Sciences, University of Hull, Hull, HU6 7RX, UK. Email: [email protected] 236 Marlborough Avenue, Hessle, HU13 0PN, UK. Email: [email protected] The apparent scarcity of many leafmining moths in East Yorkshire (see Sutton & Beaumont, 1989) is at least partly due to a lack of recorder effort, and a number of previously presumed scarce or rare moths are actually relatively widespread (Chesmore, 2008; Nunn, 2015). One of the aims of a previous article (Nunn, op. cit.) was, hopefully, to encourage searches for leafminers in an attempt to redress the imbalance of records in Yorkshire. This article summarises our 'leafminering' highlights from 2015. A number of sites in VC61 were searched for leafmining moths (Table 1). Sampling effort varied considerably between sites. AN’s ‘leafminering’ opportunities were mostly restricted to casual observations while on family outings; BW’s focussed on sites that could be reached using public transport. The most time was spent in the Hessle area and a productive site in Hull. In October, the authors joined Charlie Fletcher and Ian Marshall for a day in an under- recorded 10km square, concentrating on the Settrington area. North Cliffe Wood was visited only in late spring and early summer, and other sites were visited briefly in the autumn. Table 1. Most notable records by the authors of leafmining moths -

Additions, Deletions and Corrections to An
Bulletin of the Irish Biogeographical Society No. 36 (2012) ADDITIONS, DELETIONS AND CORRECTIONS TO AN ANNOTATED CHECKLIST OF THE IRISH BUTTERFLIES AND MOTHS (LEPIDOPTERA) WITH A CONCISE CHECKLIST OF IRISH SPECIES AND ELACHISTA BIATOMELLA (STAINTON, 1848) NEW TO IRELAND K. G. M. Bond1 and J. P. O’Connor2 1Department of Zoology and Animal Ecology, School of BEES, University College Cork, Distillery Fields, North Mall, Cork, Ireland. e-mail: <[email protected]> 2Emeritus Entomologist, National Museum of Ireland, Kildare Street, Dublin 2, Ireland. Abstract Additions, deletions and corrections are made to the Irish checklist of butterflies and moths (Lepidoptera). Elachista biatomella (Stainton, 1848) is added to the Irish list. The total number of confirmed Irish species of Lepidoptera now stands at 1480. Key words: Lepidoptera, additions, deletions, corrections, Irish list, Elachista biatomella Introduction Bond, Nash and O’Connor (2006) provided a checklist of the Irish Lepidoptera. Since its publication, many new discoveries have been made and are reported here. In addition, several deletions have been made. A concise and updated checklist is provided. The following abbreviations are used in the text: BM(NH) – The Natural History Museum, London; NMINH – National Museum of Ireland, Natural History, Dublin. The total number of confirmed Irish species now stands at 1480, an addition of 68 since Bond et al. (2006). Taxonomic arrangement As a result of recent systematic research, it has been necessary to replace the arrangement familiar to British and Irish Lepidopterists by the Fauna Europaea [FE] system used by Karsholt 60 Bulletin of the Irish Biogeographical Society No. 36 (2012) and Razowski, which is widely used in continental Europe. -

Dermatological Remedies in the Traditional Pharmacopoeia of Vulture-Alto Bradano, Inland Southern Italy Cassandra L
Florida International University FIU Digital Commons Department of Biological Sciences College of Arts, Sciences & Education 2-6-2008 Dermatological remedies in the traditional pharmacopoeia of Vulture-Alto Bradano, inland southern Italy Cassandra L. Quave Department of Biological Sciences, Florida International University, [email protected] Andrea Pieroni University of Bradford Bradley C. Bennett Department of Biological Sciences, Florida International University, [email protected] Follow this and additional works at: https://digitalcommons.fiu.edu/cas_bio Part of the Biology Commons Recommended Citation Quave, Cassandra L.; Pieroni, Andrea; and Bennett, Bradley C., "Dermatological remedies in the traditional pharmacopoeia of Vulture-Alto Bradano, inland southern Italy" (2008). Department of Biological Sciences. 157. https://digitalcommons.fiu.edu/cas_bio/157 This work is brought to you for free and open access by the College of Arts, Sciences & Education at FIU Digital Commons. It has been accepted for inclusion in Department of Biological Sciences by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. Journal of Ethnobiology and Ethnomedicine BioMed Central Research Open Access Dermatological remedies in the traditional pharmacopoeia of Vulture-Alto Bradano, inland southern Italy Cassandra L Quave*1, Andrea Pieroni2 and Bradley C Bennett1 Address: 1Department of Biological Sciences and Center for Ethnobiology and Natural Products, Florida International University, 11200 SW 8th St., HLS -
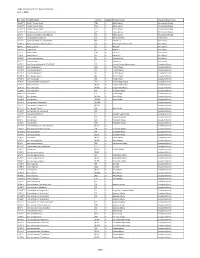
UDBG Inventory of Tree and and Shrubs June 1, 2018 Page 1
UDBG Inventory of Tree and and Shrubs June 1, 2018 Acc. Num Scientific Name Location Mapped Common Name Family Common Name 15-167*3 Abelia 'Canyon Creek' TE2 Y Glossy Abelia Honeysuckle Family 15-167*2 Abelia 'Canyon Creek' TE2 Y Glossy Abelia Honeysuckle Family 15-167*1 Abelia 'Canyon Creek' TE2 Y Glossy Abelia Honeysuckle Family 01-144*1 Abelia x grandiflora [Confetti] = 'Conti' GH Y Glossy Abelia Honeysuckle Family 02-2*1 Abelia x grandiflora 'Little Richard' F5 N Glossy Abelia Honeysuckle Family 70-1*1 Abeliophyllum distichum C3 N White Forsythia Olive Family 15-57*1 Abies balsamea var. phanerolepis NBF Y Balsam Fir Pine Family 90-47*1 Abies cephalonica 'Meyer's Dwarf' C2 N Meyer's Dwarf Grecian Fir Pine Family 89-4*1 Abies concolor C1 N White Fir Pine Family 01-74*1 Abies firma C1 N Momi Fir Pine Family 12-9*7 Abies fraseri PILL N Fraser Fir Pine Family 71-1*2 Abies koreana C1 N Korean Fir Pine Family 95-26*1 Abies nordmanniana C1 N Nordmann Fir Pine Family 96-21*1 Abies pinsapo C1 N Spanish Fir Pine Family 14-72*1 Acer [Crimson Sunset] = 'JFS-KW202' FE Y Crimson Sunset hybrid maple Soapberry Family 88-40*1 Acer buergerianum W2 N Trident Maple Soapberry Family 91-32*1 Acer buergerianum C3 N Trident Maple Soapberry Family 92-78*1 Acer buergerianum A2 Y Trident Maple Soapberry Family 92-78*2 Acer buergerianum A2 Y Trident Maple Soapberry Family 97-43*1 Acer campestre C3 N Hedge Maple Soapberry Family 94-95*1 Acer campsetre 'Compactum' WV1 N Dwarf Hedge Maple Soapberry Family 93-41*1 Acer circinatum NUR5 N Oregon Vine Maple Soapberry Family 93-41*2 Acer circinatum NUR5 N Oregon Vine Maple Soapberry Family 91-58*1 Acer cissifolium F2 Y Ivy-leafed Maple Soapberry Family 98-123*1 Acer davidii FH Y David Maple Soapberry Family 94-13*1 Acer ginnala NUR30 N Amur Maple Soapberry Family 94-13*2 Acer ginnala NUR30 N Amur Maple Soapberry Family 93-56*1 Acer ginnala 'Compactum' NUR29 N Soapberry Family 93-56*2 Acer ginnala 'Compactum' NUR29 N Soapberry Family 97-11*1 Acer ginnala 'Flame' C3 N Amur Maple Soapberry Family 97-13*1 Acer ginnala var. -

Meadow and Woodland Toolkit
Meadow & Woodland Toolkit PEOPLE FOR POLLINATORS EXISTING CONDITIONS People for Pollinators is a 8,700 sq.ft planted mead- particular) but also with regards to aesthetics and the ow surrounded by fencing, with a planted shrub visitor experience. layer on the south side of the fence, adjacent to woodland edges and open fields abutting the Lincoln LLCT’s goals for the site include expanding public Public Schools property. The site is situated on the education and programming; access to the location, northernmost portion of a 10.2-acre site owned and therefore, needs to be more clear and welcoming. protected by LLCT. The soils are mesic and nearly The meadow is currently surrounded by an 8 ft. tall all of the site is in full sun. chain link fence, with only one gate for entry, sit- uated on the northern side. The fence was initially Since 2016, LLCT has managed the site for native installed to prevent deer browse and to deter dog pollinators by direct seeding and planting a variety of forbs, graminoids and shrubs. Approximately 25- 35% of the fenced in meadow remains as non-native grasses and common weeds. After an initial survey of plant species diversity on the site by Evan Abramson and Adam Kohl of Landscape Interactions in 2019, Dr. Gegear sur- veyed the site for bumblebees and at-risk butterflies multiple times in 2020. While pollinator populations at the site were categorized as “high abundance, high diversity” by Dr. Gegear, a lot of room remains for improvement, not only in native plant species diver- sity (early season pollen sources and host plants in 38 LINCOLN POLLINATOR ACTION PLAN Off-Site Emergent Wetland Lincoln Public School Parking Lot PASTURE Path to Site walkers from allowing their dogs off leash. -

Nepticulidae from the Volga and Ural Region
Nieukerken_Nepticulidae _ final 17.12.2004 11:09 Uhr Seite 125 Nota lepid. 27 (2/3): 125–157 125 Nepticulidae from the Volga and Ural region ERIK J. VAN NIEUKERKEN1, VADIM V. Z OLOTUHIN2 & ANDREY MISTCHENKO2 1 National Museum of Natural History Naturalis PO Box 9517, NL-2300 RA Leiden, Netherlands, e-mail: [email protected] 2 Ulyanovsk State Pedagogical University, pl. 100-letiya Lenina 4, RUS-432700 Ulyanovsk, Russia, e-mail: [email protected] Abstract. The Nepticulidae of the Russian provinces (oblasts) Ul’yanovsk, Samara, Saratov, Volgograd, Astrakhan and Chelyabinsk and the Kalmyk Republic are listed. We record 60 species, including two only previously recorded, 28 species only on the basis of leafmines (indicated with an *). Seventeen species are recorded as new for Russia. Eleven of these are reported on the basis of adults: Stigmella glutinosae (Stainton, 1858), S. ulmiphaga (Preissecker, 1942), S. thuringiaca (Petry, 1904), S. rolandi Van Nieukerken, 1990, S. hybnerella (Hübner, 1813), Trifurcula (Trifurcula) subnitidella (Duponchel, 1843), T. (T.) silviae Van Nieukerken, 1990, T. (T.) beirnei Puplesis, 1984, T. (T.) chamaecytisi Z. & A. La√tüvka, 1994, Ectoedemia (Zimmermannia) liebwerdella Zimmermann, 1940 and Ectoedemia (Ectoedemia) caradjai (Groschke, 1944). Six species are reported on the basis of mines only: Stigmella freyella (Heyden, 1858), S. nivenburgensis (Preissecker, 1942), S. paradoxa (Frey, 1858), S. perpygmaeella (Doubleday, 1859), Ectoedemia (Ectoedemia) atricollis (Stainton, 1857) and E. spinosella (Joannis, 1908). Astigmella dissona Puplesis, 1984 is synonymised with Stigmella naturnella (Klimesch, 1936), here recorded for European Russia for the first time, bridging the gap between Far East Russia and Europe. S. juryi Puplesis, 1991 is synonymised with S. -

Tarset and Greystead Biological Records
Tarset and Greystead Biological Records published by the Tarset Archive Group 2015 Foreword Tarset Archive Group is delighted to be able to present this consolidation of biological records held, for easy reference by anyone interested in our part of Northumberland. It is a parallel publication to the Archaeological and Historical Sites Atlas we first published in 2006, and the more recent Gazeteer which both augments the Atlas and catalogues each site in greater detail. Both sets of data are also being mapped onto GIS. We would like to thank everyone who has helped with and supported this project - in particular Neville Geddes, Planning and Environment manager, North England Forestry Commission, for his invaluable advice and generous guidance with the GIS mapping, as well as for giving us information about the archaeological sites in the forested areas for our Atlas revisions; Northumberland National Park and Tarset 2050 CIC for their all-important funding support, and of course Bill Burlton, who after years of sharing his expertise on our wildflower and tree projects and validating our work, agreed to take this commission and pull everything together, obtaining the use of ERIC’s data from which to select the records relevant to Tarset and Greystead. Even as we write we are aware that new records are being collected and sites confirmed, and that it is in the nature of these publications that they are out of date by the time you read them. But there is also value in taking snapshots of what is known at a particular point in time, without which we have no way of measuring change or recognising the hugely rich biodiversity of where we are fortunate enough to live. -

Phytochemical Studies on the Bioactive Constituents of Hypericum Oblongifolium
PHYTOCHEMICAL STUDIES ON THE BIOACTIVE CONSTITUENTS OF HYPERICUM OBLONGIFOLIUM A thesis submitted to the University of the Punjab For the Award of Degree of Doctor of Philosophy in CHEMISTRY BY ANAM SAJID INSTITUTE OF CHEMISTRY UNIVERSITY OF THE PUNJAB LAHORE 2017 DEDICATION I Dedicate my work To my Parents My Husband And My Little Angels Ghanim and Afnan DECLARATION I, Anam Sajid d/o Sajid Saddique, solemnly declare that the thesis entitled “Phytochemical Studies on the Bioactive Constituents of Hypericum oblongifolium” has been submitted by me for the fulfillment of the requirement of the degree of Doctor of Philosophy in Chemistry at Institute of Chemistry, University of the Punjab, Lahore, under the supervision of Dr. Ejaz Ahmed and Dr. Ahsan Sharif. I also declare that the work is original unless otherwise referred or acknowledged and has never been submitted elsewhere for any other degree at any other institute. Anam Sajid Institute of Chemistry, University of the Punjab, Lahore APPROVAL CERTIFICATE It is hereby certified that this thesis is based on the results of experiments carried out by Ms. Anam Sajid and that it has not been previously presented for a higher degree elsewhere. She has done this research work under our supervision. Also we found no typographical and grammatical mistake while reviewing the thesis. She has fulfilled all requirements and is qualified to submit the accompanying thesis for the award of the degree of Doctor of Philosophy in Chemistry. Supervisors Dr. Ejaz Ahmed Institute of Chemistry, University of the Punjab, Lahore, Pakistan. Dr. Ahsan Sharif Institute of Chemistry, University of the Punjab, Lahore, Pakistan. -

Order Lepidoptera, Family Nepticulidae
Arthropod fauna of the UAE, 3: 492–514 Date of publication: 31.03.2010 Order Lepidoptera, family Nepticulidae Erik J. van Nieukerken INTRODUCTION The Nepticulidae are a family of about 800 named species of very small moths (wingspan less than 10 mm), of which the larvae make leaf-mines, stem-mines or rarely galls. The family is poorly known from the desert regions in Northern Africa and the Middle East, but relatively well known from Central Asian deserts (Turkmenistan, Uzbekistan, Mongolia), thanks to the work of R. Puplesis and students (summarised in Puplesis, 1994). The family was previously hardly known from the Arabian Peninsula, except for four species, recently described from northern Oman (Puplesis & Diškus, 2003). Here the family is recorded for the first time from the UAE, with seven species, two in Stigmella Schrank, 1802, and five in Acalyptris Meyrick, 1921, of which one is described as new. Except for S. birgittae Gustafsson, 1985, these species are also new for the Arabian Peninsula. Because some of the recorded species are actually rather common and widespread in the desert regions of North Africa and Asia, but virtually unknown in the literature, several unpublished records and synonymies of these species are presented here and they are redescribed. In this way the family Nepticulidae is not only recorded for the first time from the UAE, but also from Libya, Sudan, Egypt, Saudi Arabia and Pakistan. Stigmella omani Puplesis & Diškus, 2003, is synonymised with S. birgittae Gustafsson, 1985, S. ziziphivora Gustafsson, 1985, is synonymised with S. zizyphi Walsingham, 1911. The latter does not occur in the UAE, but is compared with the closely related S.