PDF Download
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Introduction 1. Arthur G. Green, in Discussion Following Ernest F. Ehrhardt, "Reminiscences of Dr. Caro," Chemistry and Industry 43 (1924): 561-65, on 564
Notes Introduction 1. Arthur G. Green, in discussion following Ernest F. Ehrhardt, "Reminiscences of Dr. Caro," Chemistry and Industry 43 (1924): 561-65, on 564. 2. This is mentioned by Ehrhardt, in "Reminiscences of Dr. Caro," on 561, who is probably referring to a letter from Raphael Meldola published in the London Times on 20 January 1915. Though in 1904 Meldola drew a connection between Caro's departure from England and the decline of the British dye industry, Caro is not mentioned in Meldola's letter to the Times. See also Ehrhardt, "Reminiscences of Dr. Caro," 564-65. 3. For a review of the industrial impact of Haber's ammonia synthesis, see Anthony S. Travis, "High Pressure Industrial Chemistry: The First Steps, 1909-1913, and the Impact," in Determinants in the Evolution of the European Chemical Industry, 1900-1939, eds. Anthony S. Travis, Harm G. SchrOter, Ernst Homburg, and Peter J. T. Morris (Dordrecht: Kluwer, 1998), 1-21. 4. Ehrhardt, "Reminiscences of Dr. Caro," 564. A useful study of Caro is Curt Schuster, "Heinrich Caro," in Ludwigshafener Chemiker, ed. Kurt Oberdorffer (Dusseldorf, 1960), vol. 2, 45-83. See also John J. Beer, Dictionary of Scientific Biography, s.v. Heinrich Caro; and Ernst Darmstaedter, "Heinrich Caro," in Das Buch der grossen Chemiker, ed. Giinther Bugge (Berlin: Chemie Verlag,1929), vol. 2, 298-309. The most informative of the obituaries is August Bernthsen, "Heinrich Caro," Berichte der Deutschen Chemischen Gesellschaft 45 (1912): 1987-2042. 5. Green, in discussion following Ehrhardt, "Reminiscences of Dr. Caro," on 564. 6. For the dearth of archival material on the British dye industry before around 1880, see entries in Peter J. -

A Month at BASF Fathi Habashi
Laval University From the SelectedWorks of Fathi Habashi August, 2019 A Month at BASF Fathi Habashi Available at: https://works.bepress.com/fathi_habashi/421/ A Month at BASF Introduction While working as a chemist in the Municipality of Alexandria and at the same time as a graduate student at the Faculty of Engineering, University of Alexandria, I spent my holidays in Europe. In May 1955 I visited the ACHEMA exhibition in Frankfurt am Main and participated in the technical visits organized by the conference. One of these visits was at the Badische Anilin- und Soda Fabrik known as BASF at Ludwigshafen. During the visit, I learned that the company invited graduate students from all over the world to a four weeks short course. I applied for this course. In 1957 I moved to the Faculty of Chemistry at the Technische Hochschule in Vienna and to my surprise I received the BASF invitation. It was sent to me to Alexandria and somehow it was directed to me to Vienna. As a result, September 1957 was to be spent as a guest of BASF. Ludwigshafen and BASF In 1844 Ludwig I King of Bavaria constructed an urban area on the Rhine on the opposite bank of Mannheim which became Ludwigshafen. The prosperity of Ludwigshafen is due to BASF. The company started as a Gas Works in 1861 in Mannheim by Friedrich Engelhorn (1821-1902) for street lighting for the town. Map showing the River Rhine, Mannheim on the right and Ludwigshafen and Oppau on the left The lower part of the River Rhine with some of its tributaries: Main and Neckar Friedrich Engelhorn (1821-1902) The gas works produced large amounts of tar as a byproduct which created a problem. -

Why Don't Inventors Patent?
NBER WORKING PAPER SERIES WHY DON'T INVENTORS PATENT? Petra Moser Working Paper 13294 http://www.nber.org/papers/w13294 NATIONAL BUREAU OF ECONOMIC RESEARCH 1050 Massachusetts Avenue Cambridge, MA 02138 August 2007 I wish to thank Ran Abramitzky, Tim Bresnahan, Latika Chaudhary, Avner Greif, Eric Hilt, Zorina Khan, Ken Sokoloff, and Peter Temin, as well as seminar participants at Berkeley, Boulder, MIT, Pisa, Stanford, Texas Law, and UCLA for helpful comments and the Hoover Institution for generous financial support through the National Fellows Program. Jon Casto, Irina Tallis, Alessandra Voena, and Anne Yeung provided excellent research assistance. The views expressed herein are those of the author(s) and do not necessarily reflect the views of the National Bureau of Economic Research. © 2007 by Petra Moser. All rights reserved. Short sections of text, not to exceed two paragraphs, may be quoted without explicit permission provided that full credit, including © notice, is given to the source. Why Don't Inventors Patent? Petra Moser NBER Working Paper No. 13294 August 2007 JEL No. D02,D21,D23,D62,K0,L1,L5,N0,N2,N21,N23,O3,O31,O34,O38 ABSTRACT This paper argues that the ability to keep innovations secret may be a key determinant of patenting. To test this hypothesis, the paper examines a newly-collected data set of more than 7,000 American and British innovations at four world's fairs between 1851 and 1915. Exhibition data show that the industry where an innovation is made is the single most important determinant of patenting. Urbanization, high innovative quality, and low costs of patenting also encourage patenting, but these influences are small compared with industry effects. -

Historical Group
Historical Group OCCASIONAL PAPERS No 7 Nitrogen, Novel High-Pressure Chemistry, and the German War Effort (1900-1918) Anthony S. Travis (Sidney M. Edelstein Center for the History and Philosophy of Science, Technology and Medicine, The Hebrew University of Jerusalem) The Seventh Wheeler Lecture Royal Society of Chemistry, 22 October 2014 April 2015 Introduction “The story still is told of a Minister, a member of the War Cabinet, who, finding the conversation at a certain dinner turning to the sinister menace of the submarine campaign, then at its height, and its effects especially on the Chile communications, turned to his neighbour with the enquiry: ‘Tell me, what is this nitrate they are all making such a fuss about?’” Stanley I. Levy, “The Status of Chemists and Chemistry”, in Chemistry and Industry, no. 11 (14 March 1924): 285-6. Apocryphal or not, this extract from the correspondence columns of the then new British journal Chemistry and Industry in 1924 exposes the apparent general ignorance in Britain, and also for a time in Germany, of a crucial and even desperate episode in the conduct of what became known as the First Great War. “Nitrate”, a commodity essential to the production of modern explosives employed in warfare, mainly aromatic nitro compounds such as TNT and picric acid, was common currency to all belligerents. Nevertheless outside of scientific and industrial circles the critical roles of what was in fact Chilean nitrate (Chilean saltpetre, or sodium nitrate), extracted from the mineral caliche, and the other nitrogen-containing chemicals of commerce, such as calcium cyanamide and ammonia, as sources of vast destructive power, was generally given little, if any, prominence at the start of the war in early August 1914. -
BASF History We Create Chemistry 1865 – 2015
BASF History We create chemistry 1865 – 2015 150 years BASF History We create chemistry 1865 – 2015 BASF celebrates its 150th anniversary in 2015. Discover a company history which shows how chemistry enables new ideas and solutions. Table of Contents Chronology: 1865 – 1901 16 Chronology: 1902 – 1924 28 Responsibility 35 Chronology: 1925 – 1944 44 Solutions 53 Chronology: 1945 – 1964 62 Global Presence 71 Chronology: 1965 – 1989 80 Joint Success 89 Chronology: 1990 – 2015 98 Development of BASF’s Logo 112 3 1865 – 1901 1902 – 1924 1925 – 1944 1945 – 1964 1965 – 1989 1990 – 2015 Workers operate filter presses by hand to get indigo as dry as possible at the end of the production process in 1921. 1865 – 1901 The Age of Dyes 1865 – 1901 1902 – 1924 1925 – 1944 1945 – 1964 1965 – 1989 1990 – 2015 Das Zeitalter der Farben XXXX Female workers take care of plants at the Agricultural Research Station Limburgerhof, today Agricultural Center Limburgerhof, around the year 1925. Pot experiments yield information about the influence of fertilizers on plant growth. 1902 – 1924 The Haber-Bosch Process and the Age of Fertilizers 1865 – 1901 1902 – 1924 1925 – 1944 1945 – 1964 1965 – 1989 1990 – 2015 A high-pressure reactor with vast dimensions is fitted at the Ludwigs- hafen site in 1935. BASF pioneers high-pressure technology and intro- duces it to the chemical industry. High-pressure technology becomes 1925 – 1944 increasingly characteristic of indus- trial chemistry. New High-Pressure Syntheses 1865 – 1901 1902 – 1924 1925 – 1944 1945 – 1964 1965 – 1989 1990 – 2015 1945 – 1964 From New Beginnings to the Plastic Age Since the 1960s, plastics have opened up many new areas of application. -

Historical Group Occasional Paper 7 Nitrogen, Novel High-Pressure
Historical Group OCCASIONAL PAPERS No 7 Nitrogen, Novel High-Pressure Chemistry, and the German War Effort (1900-1918) Anthony S. Travis (Sidney M. Edelstein Center for the History and Philosophy of Science, Technology and Medicine, The Hebrew University of Jerusalem) The Seventh Wheeler Lecture Royal Society of Chemistry, 22 October 2014 April 2015 Introduction “The story still is told of a Minister, a member of the War Cabinet, who, finding the conversation at a certain dinner turning to the sinister menace of the submarine campaign, then at its height, and its effects especially on the Chile communications, turned to his neighbour with the enquiry: ‘Tell me, what is this nitrate they are all making such a fuss about?’” Stanley I. Levy, “The Status of Chemists and Chemistry”, in Chemistry and Industry, no. 11 (14 March 1924): 285-6. Apocryphal or not, this extract from the correspondence columns of the then new British journal Chemistry and Industry in 1924 exposes the apparent general ignorance in Britain, and also for a time in Germany, of a crucial and even desperate episode in the conduct of what became known as the First Great War. “Nitrate”, a commodity essential to the production of modern explosives employed in warfare, mainly aromatic nitro compounds such as TNT and picric acid, was common currency to all belligerents. Nevertheless outside of scientific and industrial circles the critical roles of what was in fact Chilean nitrate (Chilean saltpetre, or sodium nitrate), extracted from the mineral caliche, and the other nitrogen-containing chemicals of commerce, such as calcium cyanamide and ammonia, as sources of vast destructive power, was generally given little, if any, prominence at the start of the war in early August 1914. -

The Laboratory
THE LABORATORY Henning Schmidgen – Bauhaus University, Weimar Suggested Citation: Henning Schmidgen, “The Laboratory,” Encyclopedia of the History of Science (April 2021) doi: 10.34758/sz06-t975 It is almost impossible to imagine science without laboratories.1 Our concept and image of modern science is fundamentally defined by special buildings in which experts utilize vast technical resources to investigate natural phenomena and processes. An entire iconography exists, depicting the laboratory scientist in the midst of extremely complex and precise instruments examining an object in his hand or looking at a brightly illuminated screen. However, the notion of the laboratory on which this iconography is based is being called into question by current developments in scientific practice. In particular, the massive research centres for particle physics, such as Fermilab near Chicago or CERN in Geneva, and the large scientific projects of recent biological research, such as the Human Genome Project, have contributed to the expansion of the laboratory into a network and its extension far beyond the confines of the natural sciences. Enhanced by the process of digitization these developments have led to a state of things in which the laboratory bears little resemblance to the traditional image of table-top experiments in an enclosed space. Nonetheless, there is no doubt that the architecturally delimited laboratory – like the factory, the railway station or the department store – is an exemplary site of modernity.2 During the last third of the 19th century in particular, specially designed and equipped buildings became central institutions of scientific endeavour. Being involved in this endeavor no longer meant striving for the formation of individual knowledge and personality, as was the case in the Romantic period. -

Book Reviews
54 Bull. Hist. Chem., VOLUME 28, Number 1 (2003) BOOK REVIEWS Methods and Styles in the Development of Chemistry. are also some caveats to that recommendation, though I Joseph S. Fruton, American Philosophical Society, Phila- am loath to place the responsibility for these solely on delphia, PA, 2002; xviii + 332 pp, Cloth, ISBN 0-87169- Dr. Fruton and suspect that many of them are due to the 245-7; $40. publisher and editors since they apply equally well to much of the recent literature in the field. Winner of the 1993 Dexter Award in the History of Chemistry, Dr. Fruton is best known for his work on the The first deals with the book’s title. There was a history of biochemistry and, more recently, for his es- time when academic books had honest titles which suc- says and autobiography. This small, attractive volume cinctly summarized their contents and intended use, such represents his first venture into the general history of as “A Short History of Chemistry”, “An Introductory chemistry and is very much in the tradition of the well- History of Chemistry”, etc., but starting in the 1970s it known short histories of chemistry by Partington and became fashionable to base titles either on catchy “in Leicester, both of which are still available as quality the know” phrases (e.g., “Atoms and Powers”) or gran- Dover paperbacks. Like its predecessors, the book tends diose higher historical or cultural themes (e.g., “Enlight- to emphasize the lives of famous chemists and, like them, enment Science in the Romantic Era”) with only the its coverage of 20th-century developments after 1925 is subtitles following the colon giving any real concrete minimal at best. -
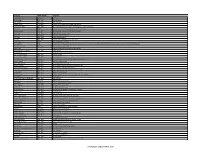
List of Famous Chemists in the World
Scientist Birth-Death Country Emil AbMderhalden 1877–1950 Swiss chemist Richard Abegg 1869–1910 German chemist Frederick Abel 1827–1902 English chemist Friedrich Accum 1769–1838 German chemist advances in the field of gas lighting Homer Burton Adkins 1892–1949 American chemist known for work in hydrogenation of organic compounds Peter Agre 1949– American chemist and doctor 2003 Nobel Prize in Chemistry Georgius Agricola 1494–1555 German scholar known as "the father of mineralogy"" Arthur Aikin 1773–1855 English chemist and mineralogist Adrien Albert 1907–1989 Australian medicinal chemist John Albery 1936–2013 English physical chemist Kurt Alder 1902–1958 German chemist 1950 Nobel Prize in Chemistry Sidney Altman 1939– 1989 Nobel Prize in Chemistry Faiza Al-Kharafi 1946– Kuwaiti chemist and academic. She was the president of Kuwait University from 1993 to 2002 and the first woman to head a major university in the Middle East. Christian B. Anfinsen 1916–1995 1972 Nobel Prize in Chemistry Angelo Angeli Brazilian Professor of Chemistry and Pharmaceutics Octavio Augusto Ceva Antunes 1731–1810 British scientist Anthony Joseph Joseph Arduengo III American chemist Johan August Arfwedson 1792–1841 Swedish chemist Anton Eduard van Arkel 1893–1976 Dutch chemist Svante Arrhenius 1859–1927 Swedish chemist one of the founders of physical chemistry Larned B. Asprey 1919–2005 American nuclear chemist Francis William Aston 1877–1945 1922 Nobel Prize in Chemistry Amedeo Avogadro 1776–1856 Italian chemist and physicist discovered Avogadro's law Stephen Moulton Babcock 1843–1931 worked on the "single-grain experiment" Werner Emmanuel Bachmann 1901–1951 American chemist known for work in steroids and RDX Leo Baekeland 1863–1944 Belgian-American chemist Adolf von Baeyer 1835–1917 German chemist 1905 Nobel Prize in Chemistry synthesis of indigo Piero Baglioni Italian chemist Hendrik Willem Bakhuis Roozeboom 1854–1907 Dutch chemist Allen J. -

Volume 79, No. 4, October 2015
Inside Volume 79, No.4, October 2015 Articles and Features 172 The simultaneous determination of hydroxyproline and pentosidine in avian skin tissue Matthew R. Campion 177 The analysis of polyphenolic compounds in selected turmeric, green tea and fruit products as a measure of potency and authenticity Darren A. Saunders, Shirley Jones, Sophia Baumann, Ellen Ashmore 184 RACI National Congress R Janadari Kariyawasam 185 Obituary: Wilfred E. (Bunt) Dasent (1926-2015) 186 IUPAC and nomenclature: brief guides and 2D (QR) InChI barcodes Richard Hartshorn 191 Trends in technological use and social relevance of New Zealand’s geothermal waters Peter Hodder 198 Employing new chemistry graduates – some thoughts from the field Michael Gray 199 Obituary: Joan Muriel (Mattingley) Cameron, Hon. FNZIC (1926-2015) 200 Some Unremembered Chemists: Heinrich Caro (1834-1910) Brian Halton 212 Obituary: Dr Alistair Campbell Other Columns 164 Comment from the President 207 Dates of Note 164 From the Editor 210 Patent Proze 165 October News 212 Subject index 171 Conference Calendar 213 Author index 171 Notice of the NZIC AGM 163 Chemistry in New Zealand October 2015 Comment from the President This issue I’d like to com- Zealand but whose vision was not accepted by the fund- ment on three themes of ing agencies. There is no ‘plan B’ for such research teams specific and current im- and once they are they dispersed, they cannot be reas- portance to our profes- sembled. The options at an individual level are few. Some sion in New Zealand. will retrain, often in fields distant from their core skills. Those who are more mobile or motivated may leave New In our July issue I noted Zealand. -

BASF Historical Milestones 1865-2005
BASF Historical Milestones 1865 - 2005 CChronik-E_1865-1900_K2.inddhronik-E_1865-1900_K2.indd 2-32-3 001.09.20051.09.2005 14:24:0214:24:02 UhrUhr The Birth of the Chemical Industry and the Era of Dyes The first stage of German indus- Soda ash has helped Britain to These efforts are strengthened trialization begins in 1835 with build the world’s most productive by calls for improved medical the building of the first German textile industry and the best care by the Berlin physician railway. At around the same time, glass industry. Soda means soap Rudolf Virchow. Experimenting customs barriers between the can be manufactured more with coal tar in 1856 in an individual German states are cheaply. Soap, once a luxury, attempt to synthesize the anti- abolished, creating an internal becomes a basic commodity. malaria drug quinine from coal German market – a powerful Thanks to improved hygiene, the tar, the young English chemist incentive for the growth of new incidence of infectious diseases William Henry Perkin obtains industries such as engineering declines rapidly and average life the first synthetic coal tar dye, and iron smelting. expectancy doubles. Soda ash aniline purple, named mauveine. becomes a significant object of Within a short time, chemists At the same time, the German global trade. throughout Europe discover a textile industry, and in particular whole range of new synthetic the mechanized processing of But the textile industry needs dyes from aniline yellow through cotton, begins to grow rapidly. dyes too. Existing natural dyes Bismarck brown to Hofmann Traditional processing methods can no longer satisfy growing violet. -

150Th Anniversary of Mauve and Coal-Tar Dyes
150th Anniversary of Mauve and Coal-Tar Dyes Anthony S. Travis, Sidney M. Edelstein Center, The Hebrew University of Jerusalem, Givat Ram, Jerusalem 91904 / Leo Baeck Institute, London <[email protected]> One-hundred-and-fifty years ago, in March 1856, a teenaged chemical inventor in London discovered in his home laboratory a process that converted aniline, made from coal-tar benzene, into a purple colorant, later known as mauve. The young man was William Henry Perkin, assistant to the German chemist August Wilhelm Hofmann, then head of the Royal College of Chemistry. From Perkin's single serendipitous discovery coal-tar products went on to become a group of organic compounds that would make tremendous contributions to material well- being, not only though synthetic dyestuffs, but pharmaceuticals, products for the processing of rubber, and new polymers. They revolutionized the study of chem- istry, and forged academic-industrial collaborations. The enterprise made reputa- tions and attracted the greatest stars of organic chemistry, including Hofmann, Adolf Baeyer, and Emil Fischer. No less profound were the economic and politi- cal consequences. The new colorants, as agents of modernity, forced changes in patent law, fostered technology transfer, stimulated the emergence of the modern chemical industry and decimated cultivation of dye yielding plants. Colorists and Chemists During the last quarter of the eighteenth century the introduction of power-driven machines for spinning, weaving and printing introduced vast changes in the pro- duction of textiles. This created unprecedented demand for dyes and pigments, or colorants, that were applied to textile goods. The supply and efficient application of colorants was critical to the success of the whole endeavor.