Identification of Two Signaling Submodules Within the Crkii/ELMO
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dock3 Protects Myelin in the Cuprizone Model for Demyelination
Citation: Cell Death and Disease (2014) 5, e1395; doi:10.1038/cddis.2014.357 OPEN & 2014 Macmillan Publishers Limited All rights reserved 2041-4889/14 www.nature.com/cddis Dock3 protects myelin in the cuprizone model for demyelination K Namekata1, A Kimura1, C Harada1, H Yoshida2, Y Matsumoto1 and T Harada*,1,2 Dedicator of cytokinesis 3 (Dock3) belongs to an atypical family of the guanine nucleotide exchange factors. It is predominantly expressed in the neural tissues and causes cellular morphological changes by activating the small GTPase Rac1. We previously reported that Dock3 overexpression protects retinal ganglion cells from excitotoxic cell death. Oligodendrocytes are the myelinating cells of axons in the central nervous system and these cells are damaged in demyelinating disorders including multiple sclerosis (MS) and optic neuritis. In this study, we examined if Dock3 is expressed in oligodendrocytes and if increasing Dock3 signals can suppress demyelination in a cuprizone-induced demyelination model, an animal model of MS. We demonstrate that Dock3 is expressed in oligodendrocytes and Dock3 overexpression protects myelin in the corpus callosum following cuprizone treatment. Furthermore, we show that cuprizone demyelinates optic nerves and the extent of demyelination is ameliorated in mice overexpressing Dock3. Cuprizone treatment impairs visual function, which was demonstrated by multifocal electroretinograms, an established non-invasive method, and Dock3 overexpression prevented this effect. In mice overexpressing Dock3, Erk activation is increased, suggesting this may at least partly explain the observed protective effects. Our findings suggest that Dock3 may be a therapeutic target for demyelinating disorders including optic neuritis. Cell Death and Disease (2014) 5, e1395; doi:10.1038/cddis.2014.357; published online 28 August 2014 Dedicator of cytokinesis 3 (Dock3), an atypical member of the will restore the visual function. -

Structure of the Dock2âˆ'elmo1 Complex Provides Insights Into
ARTICLE https://doi.org/10.1038/s41467-020-17271-9 OPEN Structure of the DOCK2−ELMO1 complex provides insights into regulation of the auto-inhibited state Leifu Chang1,7, Jing Yang1, Chang Hwa Jo 2, Andreas Boland 1,8, Ziguo Zhang 1, Stephen H. McLaughlin 1, Afnan Abu-Thuraia 3, Ryan C. Killoran2, Matthew J. Smith 2,4,9, Jean-Francois Côté 3,5,6,9 & ✉ David Barford 1 DOCK (dedicator of cytokinesis) proteins are multidomain guanine nucleotide exchange 1234567890():,; factors (GEFs) for RHO GTPases that regulate intracellular actin dynamics. DOCK proteins share catalytic (DOCKDHR2) and membrane-associated (DOCKDHR1) domains. The structurally-related DOCK1 and DOCK2 GEFs are specific for RAC, and require ELMO (engulfment and cell motility) proteins for function. The N-terminal RAS-binding domain (RBD) of ELMO (ELMORBD) interacts with RHOG to modulate DOCK1/2 activity. Here, we determine the cryo-EM structures of DOCK2−ELMO1 alone, and as a ternary complex with RAC1, together with the crystal structure of a RHOG−ELMO2RBD complex. The binary DOCK2−ELMO1 complex adopts a closed, auto-inhibited conformation. Relief of auto- inhibition to an active, open state, due to a conformational change of the ELMO1 subunit, exposes binding sites for RAC1 on DOCK2DHR2, and RHOG and BAI GPCRs on ELMO1. Our structure explains how up-stream effectors, including DOCK2 and ELMO1 phosphorylation, destabilise the auto-inhibited state to promote an active GEF. 1 MRC Laboratory of Molecular Biology, Cambridge CB2 0QH, UK. 2 Institute for Research in Immunology and Cancer, Université de Montréal, Montréal, Québec H3T 1J4, Canada. 3 Montreal Institute of Clinical Research (IRCM), Montréal, QC H2W 1R7, Canada. -
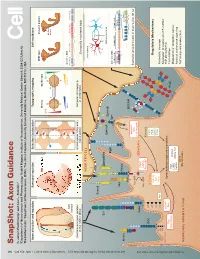
Snapshot: Axon Guidance Pasterkamp R
494 1 Cell Cell ??? SnapShot: Axon Guidance 153 SnapShot: XXXXXXXXXXXXXXXXXXXXXXXXXX 1 2 , ??MONTH?? ??DATE??, 200? ©200? Elsevier Inc. 200?©200? ElsevierInc. , ??MONTH?? ??DATE??, DOI R. Jeroen Pasterkamp and Alex L. Kolodkin , April11, 2013©2013Elsevier Inc. DOI http://dx.doi.org/10.1016/j.cell.2013.03.031 AUTHOR XXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXX 1 AFFILIATIONDepartment of XXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXX Neuroscience and Pharmacology, Rudolf Magnus Institute of Neuroscience, University Medical Center Utrecht, 3584 CG Utrecht, The Netherlands; 2Department of Neuroscience, HHMI, The Johns Hopkins University School of Medicine, Baltimore, MD 21212, USA Axon attraction and repulsion Surround repulsion Selective fasciculation Topographic mapping Self-avoidance Wild-type Dscam1 mutant Sema3A A Retina SC/Tectum EphB EphrinB P D D Mutant T A neuron N P A V V Genomic DNA P EphA EphrinA Slit Exon 4 (12) Exon 6 (48) Exon 9 (33) Exon 17 (2) Netrin Commissural axon guidance Surround repulsion of peripheral Grasshopper CNS axon Retinotectal mapping at the CNS midline nerves in vertebrates fasciculation in vertebrates Drosophila mushroom body XXXXXXXXX NEURITE/CELL Isoneuronal Sema3 Slit Sema1/4-6 Heteroneuronal EphrinA EphrinB FasII Eph Genomic DNA Pcdh-α (14) Pcdh-β (22) Pcdh-γ (22) Variable Con Variable Con Nrp Plexin ** *** Netrin LAMELLIPODIA Con DSCAM ephexin Starburst amacrine cells in mammalian retina Ras-GTP Vav See online version for legend and references. α-chimaerin GEFs/GAPs Robo FARP Ras-GDP LARG RhoGEF Kinases DCC cc0 GTPases PKA cc1 FAK Regulatory Mechanisms See online versionfor??????. Cdc42 GSK3 cc2 Rac PI3K P1 Rho P2 cc3 Abl Proteolytic cleavage P3 Regulation of expression (TF, miRNA, FILOPODIA srGAP Cytoskeleton regulatory proteins multiple isoforms) Sos Trio Pcdh Cis inhibition DOCK180 PAK ROCK Modulation of receptors’ output LIMK Myosin-II Colin Forward and reverse signaling Actin Trafcking and endocytosis NEURONAL GROWTH CONE Microtubules SnapShot: Axon Guidance R. -

Pi3kp110-, Src-, FAK-Dependent and DOCK2-Independent Migration and Invasion of CXCL13-Stimulated Prostate Cancer Cells
El Haibi et al. Molecular Cancer 2010, 9:85 http://www.molecular-cancer.com/content/9/1/85 RESEARCH Open Access PI3Kp110-,Research Src-, FAK-dependent and DOCK2-independent migration and invasion of CXCL13-stimulated prostate cancer cells Christelle P El Haibi1, Praveen K Sharma2, Rajesh Singh3, Paul R Johnson4, Jill Suttles2, Shailesh Singh3 and James W Lillard Jr*3 Abstract Background: Most prostate cancer (PCa)-related deaths are due to metastasis, which is mediated in part by chemokine receptor and corresponding ligand interaction. We have previously shown that PCa tissue and cell lines express high levels of the chemokine receptor CXCR5, than compared to their normal counterparts, and interaction of CXCR5 with its specific ligand (CXCL13) promoted PCa cell invasion, migration, and differential matrix metalloproteinase (MMP) expression. This study dissects some of the molecular mechanisms following CXCL13-CXCR5 interaction that mediate PCa cell migration and invasion. Results: Using Western blot analysis, kinase-specific cell-based ELISAs, and migration and invasion assays, we show that PCa cell lines differentially express phosphoinositide-3 kinase (PI3K) catalytic subunit isoforms and dedicator of cytokinesis 2 (DOCK2). Specifically, we show that PC3 and normal prostatic epithelial (RWPE-1), but not LNCaP cell lines expressed DOCK2, while RWPE, PC3, and LNCaP cell lines expressed PI3K-p110α and -p110β. Moreover, PC3 selectively expressed PI3K-p110γ, but LNCaP and RWPE cell lines expressed PI3Kp110δ. CXCL13 caused CXCR5-dependent activation of the PI3Kp85α in LNCaP cells, and p85α as well as -p101 in PC3 cells. CXCL13-CXCR5 interaction regulated LNCaP and PC3 cell migration and invasion through extracellular signal-regulated kinase 1/2 (ERK1/2) activation that was primarily dependent on the PI3Kp110 isoform(s), Src, and focal adhesion kinase (FAK), but not DOCK2. -

Regulation of the Mammalian Target of Rapamycin Complex 2 (Mtorc2)
Regulation of the Mammalian Target Of Rapamycin Complex 2 (mTORC2) Inauguraldissertation Zur Erlangung der Würde eines Doktors der Philosophie vorgelegt der Philosophisch-Naturwissenschaftlichen Fakultät der Universität Basel von Klaus-Dieter Molle aus Heilbronn, Deutschland Basel, 2006 Genehmigt von der Philosophisch-Naturwissenschaftlichen Fakultät Auf Antrag von Prof. Michael N. Hall und Prof. Markus Affolter. Basel, den 21.11.2006 Prof. Hans-Peter Hauri Dekan Summary The growth controlling mammalian Target of Rapamycin (mTOR) is a conserved Ser/Thr kinase found in two structurally and functionally distinct complexes, mTORC1 and mTORC2. The tumor suppressor TSC1-TSC2 complex inhibits mTORC1 by acting on the small GTPase Rheb, but the role of TSC1-TSC2 and Rheb in the regulation of mTORC2 is unclear. Here we examined the role of TSC1-TSC2 in the regulation of mTORC2 in human embryonic kidney 293 cells. Induced knockdown of TSC1 and TSC2 (TSC1/2) stimulated mTORC2-dependent actin cytoskeleton organization and Paxillin phosphorylation. Furthermore, TSC1/2 siRNA increased mTORC2-dependent Ser473 phosphorylation of plasma membrane bound, myristoylated Akt/PKB. This suggests that loss of Akt/PKB Ser473 phosphorylation in TSC mutant cells, as reported previously, is due to inhibition of Akt/PKB localization rather than inhibition of mTORC2 activity. Amino acids and overexpression of Rheb failed to stimulate mTORC2 signaling. Thus, TSC1-TSC2 also inhibits mTORC2, but possibly independently of Rheb. Our results suggest that mTORC2 hyperactivation may contribute to the pathophysiology of diseases such as cancer and Tuberous Sclerosis Complex. i Acknowledgement During my PhD studies in the Biozentrum I received a lot of support from many people around me who I mention here to express my gratefulness. -

CXCL13/CXCR5 Interaction Facilitates VCAM-1-Dependent Migration in Human Osteosarcoma
International Journal of Molecular Sciences Article CXCL13/CXCR5 Interaction Facilitates VCAM-1-Dependent Migration in Human Osteosarcoma 1, 2,3,4, 5 6 7 Ju-Fang Liu y, Chiang-Wen Lee y, Chih-Yang Lin , Chia-Chia Chao , Tsung-Ming Chang , Chien-Kuo Han 8, Yuan-Li Huang 8, Yi-Chin Fong 9,10,* and Chih-Hsin Tang 8,11,12,* 1 School of Oral Hygiene, College of Oral Medicine, Taipei Medical University, Taipei City 11031, Taiwan; [email protected] 2 Department of Orthopaedic Surgery, Chang Gung Memorial Hospital, Puzi City, Chiayi County 61363, Taiwan; [email protected] 3 Department of Nursing, Division of Basic Medical Sciences, and Chronic Diseases and Health Promotion Research Center, Chang Gung University of Science and Technology, Puzi City, Chiayi County 61363, Taiwan 4 Research Center for Industry of Human Ecology and Research Center for Chinese Herbal Medicine, Chang Gung University of Science and Technology, Guishan Dist., Taoyuan City 33303, Taiwan 5 School of Medicine, China Medical University, Taichung 40402, Taiwan; [email protected] 6 Department of Respiratory Therapy, Fu Jen Catholic University, New Taipei City 24205, Taiwan; [email protected] 7 School of Medicine, Institute of Physiology, National Yang-Ming University, Taipei City 11221, Taiwan; [email protected] 8 Department of Biotechnology, College of Health Science, Asia University, Taichung 40402, Taiwan; [email protected] (C.-K.H.); [email protected] (Y.-L.H.) 9 Department of Sports Medicine, College of Health Care, China Medical University, Taichung 40402, Taiwan 10 Department of Orthopedic Surgery, China Medical University Beigang Hospital, Yunlin 65152, Taiwan 11 Department of Pharmacology, School of Medicine, China Medical University, Taichung 40402, Taiwan 12 Chinese Medicine Research Center, China Medical University, Taichung 40402, Taiwan * Correspondence: [email protected] (Y.-C.F.); [email protected] (C.-H.T.); Tel.: +886-4-2205-2121-7726 (C.-H.T.); Fax: +886-4-2233-3641 (C.-H.T.) These authors contributed equally to this work. -

The Atypical Guanine-Nucleotide Exchange Factor, Dock7, Negatively Regulates Schwann Cell Differentiation and Myelination
The Journal of Neuroscience, August 31, 2011 • 31(35):12579–12592 • 12579 Cellular/Molecular The Atypical Guanine-Nucleotide Exchange Factor, Dock7, Negatively Regulates Schwann Cell Differentiation and Myelination Junji Yamauchi,1,3,5 Yuki Miyamoto,1 Hajime Hamasaki,1,3 Atsushi Sanbe,1 Shinji Kusakawa,1 Akane Nakamura,2 Hideki Tsumura,2 Masahiro Maeda,4 Noriko Nemoto,6 Katsumasa Kawahara,5 Tomohiro Torii,1 and Akito Tanoue1 1Department of Pharmacology and 2Laboratory Animal Resource Facility, National Research Institute for Child Health and Development, Setagaya, Tokyo 157-8535, Japan, 3Department of Biological Sciences, Tokyo Institute of Technology, Midori, Yokohama 226-8501, Japan, 4IBL, Ltd., Fujioka, Gumma 375-0005, Japan, and 5Department of Physiology and 6Bioimaging Research Center, Kitasato University School of Medicine, Sagamihara, Kanagawa 252-0374, Japan In development of the peripheral nervous system, Schwann cells proliferate, migrate, and ultimately differentiate to form myelin sheath. In all of the myelination stages, Schwann cells continuously undergo morphological changes; however, little is known about their underlying molecular mechanisms. We previously cloned the dock7 gene encoding the atypical Rho family guanine-nucleotide exchange factor (GEF) and reported the positive role of Dock7, the target Rho GTPases Rac/Cdc42, and the downstream c-Jun N-terminal kinase in Schwann cell migration (Yamauchi et al., 2008). We investigated the role of Dock7 in Schwann cell differentiation and myelination. Knockdown of Dock7 by the specific small interfering (si)RNA in primary Schwann cells promotes dibutyryl cAMP-induced morpholog- ical differentiation, indicating the negative role of Dock7 in Schwann cell differentiation. It also results in a shorter duration of activation of Rac/Cdc42 and JNK, which is the negative regulator of myelination, and the earlier activation of Rho and Rho-kinase, which is the positive regulator of myelination. -

A Rac/Cdc42 Exchange Factor Complex Promotes Formation of Lateral filopodia and Blood Vessel Lumen Morphogenesis
ARTICLE Received 1 Oct 2014 | Accepted 26 Apr 2015 | Published 1 Jul 2015 DOI: 10.1038/ncomms8286 OPEN A Rac/Cdc42 exchange factor complex promotes formation of lateral filopodia and blood vessel lumen morphogenesis Sabu Abraham1,w,*, Margherita Scarcia2,w,*, Richard D. Bagshaw3,w,*, Kathryn McMahon2,w, Gary Grant2, Tracey Harvey2,w, Maggie Yeo1, Filomena O.G. Esteves2, Helene H. Thygesen2,w, Pamela F. Jones4, Valerie Speirs2, Andrew M. Hanby2, Peter J. Selby2, Mihaela Lorger2, T. Neil Dear4,w, Tony Pawson3,z, Christopher J. Marshall1 & Georgia Mavria2 During angiogenesis, Rho-GTPases influence endothelial cell migration and cell–cell adhesion; however it is not known whether they control formation of vessel lumens, which are essential for blood flow. Here, using an organotypic system that recapitulates distinct stages of VEGF-dependent angiogenesis, we show that lumen formation requires early cytoskeletal remodelling and lateral cell–cell contacts, mediated through the RAC1 guanine nucleotide exchange factor (GEF) DOCK4 (dedicator of cytokinesis 4). DOCK4 signalling is necessary for lateral filopodial protrusions and tubule remodelling prior to lumen formation, whereas proximal, tip filopodia persist in the absence of DOCK4. VEGF-dependent Rac activation via DOCK4 is necessary for CDC42 activation to signal filopodia formation and depends on the activation of RHOG through the RHOG GEF, SGEF. VEGF promotes interaction of DOCK4 with the CDC42 GEF DOCK9. These studies identify a novel Rho-family GTPase activation cascade for the formation of endothelial cell filopodial protrusions necessary for tubule remodelling, thereby influencing subsequent stages of lumen morphogenesis. 1 Institute of Cancer Research, Division of Cancer Biology, 237 Fulham Road, London SW3 6JB, UK. -

Tyr724 Phosphorylation of ELMO1 by Src Is Involved in Cell Spreading and Migration Via Rac1 Activation
Title Tyr724 phosphorylation of ELMO1 by Src is involved in cell spreading and migration via Rac1 activation Makino, Yoshinori; Tsuda, Masumi; Ohba, Yusuke; Nishihara, Hiroshi; Sawa, Hirofumi; Nagashima, Kazuo; Tanaka, Author(s) Shinya Cell communication and signaling, 13, 35 Citation https://doi.org/10.1186/s12964-015-0113-y Issue Date 2015-07-26 Doc URL http://hdl.handle.net/2115/59828 Rights(URL) http://creativecommons.org/licenses/by/4.0 Type article File Information s12964-015-0113-y.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP Makino et al. Cell Communication and Signaling (2015) 13:35 DOI 10.1186/s12964-015-0113-y RESEARCH ARTICLE Open Access Tyr724 phosphorylation of ELMO1 by Src is involved in cell spreading and migration via Rac1 activation Yoshinori Makino1,2, Masumi Tsuda1, Yusuke Ohba3, Hiroshi Nishihara4, Hirofumi Sawa1,5, Kazuo Nagashima1,6 and Shinya Tanaka1,4* Abstract Background: The complex of Dock180/ELMO1 that functions as a bipartite guanine nucleotide exchange factor for Rac is essential for diverse physiological and pathological processes of cells such as cell migration, phagocytosis, and invasion of cancer cells. Among the Src-family tyrosine kinases (SFKs), it has been reported that Hck directly phosphorylates ELMO1, regulating phagocytosis by promoting activation of Rac1; however, the involvement of other SFKs in ELMO1 phosphorylation has remained unknown. Here, we identified novel tyrosine (Y) residues of ELMO1 phosphorylated by SFKs, and examined the effects on Rac1 activity, cell adhesion, spreading, and cell motility on extracellular matrix (ECM). Results: In this study, we unveiled that Src and Fyn can induce tyrosine phosphorylation of ELMO1 in in vivo and in vitro phosphorylation assays. -

Targeting PH Domain Proteins for Cancer Therapy
The Texas Medical Center Library DigitalCommons@TMC The University of Texas MD Anderson Cancer Center UTHealth Graduate School of The University of Texas MD Anderson Cancer Biomedical Sciences Dissertations and Theses Center UTHealth Graduate School of (Open Access) Biomedical Sciences 12-2018 Targeting PH domain proteins for cancer therapy Zhi Tan Follow this and additional works at: https://digitalcommons.library.tmc.edu/utgsbs_dissertations Part of the Bioinformatics Commons, Medicinal Chemistry and Pharmaceutics Commons, Neoplasms Commons, and the Pharmacology Commons Recommended Citation Tan, Zhi, "Targeting PH domain proteins for cancer therapy" (2018). The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences Dissertations and Theses (Open Access). 910. https://digitalcommons.library.tmc.edu/utgsbs_dissertations/910 This Dissertation (PhD) is brought to you for free and open access by the The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences at DigitalCommons@TMC. It has been accepted for inclusion in The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences Dissertations and Theses (Open Access) by an authorized administrator of DigitalCommons@TMC. For more information, please contact [email protected]. TARGETING PH DOMAIN PROTEINS FOR CANCER THERAPY by Zhi Tan Approval page APPROVED: _____________________________________________ Advisory Professor, Shuxing Zhang, Ph.D. _____________________________________________ -

Crit. Rev. in Biochem. and Molec. Biol. 2011
Critical Reviews in Biochemistry and Molecular Biology Critical Reviews in Biochemistry and Molecular Biology, 2011, 1–21, Early Online 2011 © 2011 Informa Healthcare USA, Inc. ISSN 1040-9238 print/ISSN 1549-7798 online 00 DOI: 10.3109/10409238.2011.618113 00 000 REVIEW 000 18 June 2011 Mammalian TOR signaling to the AGC kinases 15 August 2011 Bing Su1 and Estela Jacinto2 24 August 2011 1Department of Immunobiology and The Vascular Biology and Therapeutics Program, Yale University School of Medicine, New Haven, CT, USA and 2Department of Physiology and Biophysics, UMDNJ-RWJMS, Piscataway, NJ, USA 1040-9238 1549-7798 Abstract The mechanistic (or mammalian) target of rapamycin (mTOR), an evolutionarily conserved protein kinase, orchestrates © 2011 Informa Healthcare USA, Inc. cellular responses to growth, metabolic and stress signals. mTOR processes various extracellular and intracellular inputs as part of two mTOR protein complexes, mTORC1 or mTORC2. The mTORCs have numerous cellular targets but 10.3109/10409238.2011.618113 members of a family of protein kinases, the protein kinase (PK)A/PKG/PKC (AGC) family are the best characterized direct mTOR substrates. The AGC kinases control multiple cellular functions and deregulation of many members of BBMG this family underlies numerous pathological conditions. mTOR phosphorylates conserved motifs in these kinases to allosterically augment their activity, influence substrate specificity, and promote protein maturation and 618113 stability. Activation of AGC kinases in turn triggers the phosphorylation of diverse, often overlapping, targets that ultimately control cellular response to a wide spectrum of stimuli. This review will highlight recent findings on how mTOR regulates AGC kinases and how mTOR activity is feedback regulated by these kinases. -

A Rhog-Mediated Signaling Pathway That Modulates Invadopodia Dynamics in Breast Cancer Cells Silvia M
© 2017. Published by The Company of Biologists Ltd | Journal of Cell Science (2017) 130, 1064-1077 doi:10.1242/jcs.195552 RESEARCH ARTICLE A RhoG-mediated signaling pathway that modulates invadopodia dynamics in breast cancer cells Silvia M. Goicoechea, Ashtyn Zinn, Sahezeel S. Awadia, Kyle Snyder and Rafael Garcia-Mata* ABSTRACT micropinocytosis, bacterial uptake, phagocytosis and leukocyte One of the hallmarks of cancer is the ability of tumor cells to invade trans-endothelial migration (deBakker et al., 2004; Ellerbroek et al., surrounding tissues and metastasize. During metastasis, cancer cells 2004; Jackson et al., 2015; Katoh et al., 2006, 2000; van Buul et al., degrade the extracellular matrix, which acts as a physical barrier, by 2007). Recent studies have revealed that RhoG plays a role in tumor developing specialized actin-rich membrane protrusion structures cell invasion and may contribute to the formation of invadopodia called invadopodia. The formation of invadopodia is regulated by Rho (Hiramoto-Yamaki et al., 2010; Kwiatkowska et al., 2012). GTPases, a family of proteins that regulates the actin cytoskeleton. Invadopodia are actin-rich adhesive structures that form in the Here, we describe a novel role for RhoG in the regulation of ventral surface of cancer cells and allow them to degrade the invadopodia disassembly in human breast cancer cells. Our results extracellular matrix (ECM) (Gimona et al., 2008). Formation of show that RhoG and Rac1 have independent and opposite roles invadopodia involves a series of steps that include the disassembly in the regulation of invadopodia dynamics. We also show that SGEF of focal adhesions and stress fibers, and the relocalization of several (also known as ARHGEF26) is the exchange factor responsible of their components into the newly formed invadopodia (Hoshino for the activation of RhoG during invadopodia disassembly.