The Deleterious Effects of Long-Term Phosphite
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Flora.Sa.Gov.Au/Jabg
JOURNAL of the ADELAIDE BOTANIC GARDENS AN OPEN ACCESS JOURNAL FOR AUSTRALIAN SYSTEMATIC BOTANY flora.sa.gov.au/jabg Published by the STATE HERBARIUM OF SOUTH AUSTRALIA on behalf of the BOARD OF THE BOTANIC GARDENS AND STATE HERBARIUM © Board of the Botanic Gardens and State Herbarium, Adelaide, South Australia © Department of Environment, Water and Natural Resources, Government of South Australia All rights reserved State Herbarium of South Australia PO Box 2732 Kent Town SA 5071 Australia © 2012 Board of the Botanic Gardens & State Herbarium, Government of South Australia J. Adelaide Bot. Gard. 25 (2012) 71–96 © 2012 Department of Environment, Water and Natural Resources, Govt of South Australia Notes on Hibbertia (Dilleniaceae) 8. Seven new species, a new combination and four new subspecies from subgen. Hemistemma, mainly from the central coast of New South Wales H.R. Toelkena & R.T. Millerb a State Herbarium of South Australia, DENR Science Resource Centre, P.O. Box 2732, Kent Town, South Australia 5071 E-mail: [email protected] b 13 Park Road, Bulli, New South Wales 2516 E-mail: [email protected] Abstract Increased collections from the Hibbertia-rich vicinity of Sydney, New South Wales, prompted a survey of rarer species to publicise the need for more information ahead of the rapid urban spread. Many of these species were previously misunderstood or are listed as rare and endangered. Thirteen new taxa (in bold) are described and discussed in context with the following seventeen taxa within seven different species groups: 1. H. acicularis group: H. woronorana Toelken; 2. H. humifusa group: H. -

Tracing History
Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 911 Tracing History Phylogenetic, Taxonomic, and Biogeographic Research in the Colchicum Family BY ANNIKA VINNERSTEN ACTA UNIVERSITATIS UPSALIENSIS UPPSALA 2003 Dissertation presented at Uppsala University to be publicly examined in Lindahlsalen, EBC, Uppsala, Friday, December 12, 2003 at 10:00 for the degree of Doctor of Philosophy. The examination will be conducted in English. Abstract Vinnersten, A. 2003. Tracing History. Phylogenetic, Taxonomic and Biogeographic Research in the Colchicum Family. Acta Universitatis Upsaliensis. Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 911. 33 pp. Uppsala. ISBN 91-554-5814-9 This thesis concerns the history and the intrafamilial delimitations of the plant family Colchicaceae. A phylogeny of 73 taxa representing all genera of Colchicaceae, except the monotypic Kuntheria, is presented. The molecular analysis based on three plastid regions—the rps16 intron, the atpB- rbcL intergenic spacer, and the trnL-F region—reveal the intrafamilial classification to be in need of revision. The two tribes Iphigenieae and Uvularieae are demonstrated to be paraphyletic. The well-known genus Colchicum is shown to be nested within Androcymbium, Onixotis constitutes a grade between Neodregea and Wurmbea, and Gloriosa is intermixed with species of Littonia. Two new tribes are described, Burchardieae and Tripladenieae, and the two tribes Colchiceae and Uvularieae are emended, leaving four tribes in the family. At generic level new combinations are made in Wurmbea and Gloriosa in order to render them monophyletic. The genus Androcymbium is paraphyletic in relation to Colchicum and the latter genus is therefore expanded. -

Flora and Vegetation Survey of the Proposed Kwinana to Australind Gas
__________________________________________________________________________________ FLORA AND VEGETATION SURVEY OF THE PROPOSED KWINANA TO AUSTRALIND GAS PIPELINE INFRASTRUCTURE CORRIDOR Prepared for: Bowman Bishaw Gorham and Department of Mineral and Petroleum Resources Prepared by: Mattiske Consulting Pty Ltd November 2003 MATTISKE CONSULTING PTY LTD DRD0301/039/03 __________________________________________________________________________________ TABLE OF CONTENTS Page 1. SUMMARY............................................................................................................................................... 1 2. INTRODUCTION ..................................................................................................................................... 2 2.1 Location................................................................................................................................................. 2 2.2 Climate .................................................................................................................................................. 2 2.3 Vegetation.............................................................................................................................................. 3 2.4 Declared Rare and Priority Flora......................................................................................................... 3 2.5 Local and Regional Significance........................................................................................................... 5 2.6 Threatened -

Great Southern Recovery Plan
Great Southern Recovery Plan The Great Southern Recovery Plan is part of the next step in our COVID-19 journey. It’s part of WA’s $5.5 billion overarching State plan, focused on building infrastructure, economic, health and social outcomes. The Great Southern Recovery Plan will deliver a pipeline of jobs in sectors including construction, manufacturing, tourism and hospitality, renewable energy, education and training, agriculture, conservation and mining. WA’s recovery is a joint effort, it’s about Government working with industry together. We managed the pandemic together as a community. Together, we will recover. Investing in our Schools and Rebuilding our TAFE Sector • $6.3 million for a new Performing Arts centre at Albany Senior High School • $1.1 million for refurbishments at North Albany Senior High School including the visual arts area and specialist subject classrooms • $17 million to South Regional TAFE’s Albany campus for new trade workshops, delivering training in the automotive, engineering and construction industries • $25 million for free TAFE short courses to upskill thousands of West Australians, with a variety of free courses available at South Regional TAFE’s Albany, Denmark, Katanning and Mount Barker campuses • $32 million to expand the Lower Fees, Local Skills program and significantly reduce TAFE fees across 39 high priority courses • $4.8 million for the Apprenticeship and Traineeship Re-engagement Incentive that provides employers with a one-off payment of $6,000 for hiring an apprentice and $3,000 for hiring -
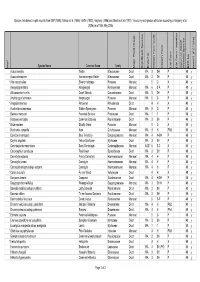
BFS048 Site Species List
Species lists based on plot records from DEP (1996), Gibson et al. (1994), Griffin (1993), Keighery (1996) and Weston et al. (1992). Taxonomy and species attributes according to Keighery et al. (2006) as of 16th May 2005. Species Name Common Name Family Major Plant Group Significant Species Endemic Growth Form Code Growth Form Life Form Life Form - aquatics Common SSCP Wetland Species BFS No kens01 (FCT23a) Wd? Acacia sessilis Wattle Mimosaceae Dicot WA 3 SH P 48 y Acacia stenoptera Narrow-winged Wattle Mimosaceae Dicot WA 3 SH P 48 y * Aira caryophyllea Silvery Hairgrass Poaceae Monocot 5 G A 48 y Alexgeorgea nitens Alexgeorgea Restionaceae Monocot WA 6 S-R P 48 y Allocasuarina humilis Dwarf Sheoak Casuarinaceae Dicot WA 3 SH P 48 y Amphipogon turbinatus Amphipogon Poaceae Monocot WA 5 G P 48 y * Anagallis arvensis Pimpernel Primulaceae Dicot 4 H A 48 y Austrostipa compressa Golden Speargrass Poaceae Monocot WA 5 G P 48 y Banksia menziesii Firewood Banksia Proteaceae Dicot WA 1 T P 48 y Bossiaea eriocarpa Common Bossiaea Papilionaceae Dicot WA 3 SH P 48 y * Briza maxima Blowfly Grass Poaceae Monocot 5 G A 48 y Burchardia congesta Kara Colchicaceae Monocot WA 4 H PAB 48 y Calectasia narragara Blue Tinsel Lily Dasypogonaceae Monocot WA 4 H-SH P 48 y Calytrix angulata Yellow Starflower Myrtaceae Dicot WA 3 SH P 48 y Centrolepis drummondiana Sand Centrolepis Centrolepidaceae Monocot AUST 6 S-C A 48 y Conostephium pendulum Pearlflower Epacridaceae Dicot WA 3 SH P 48 y Conostylis aculeata Prickly Conostylis Haemodoraceae Monocot WA 4 H P 48 y Conostylis juncea Conostylis Haemodoraceae Monocot WA 4 H P 48 y Conostylis setigera subsp. -

Introduction Methods Results
Papers and Proceedings Royal Society ofTasmania, Volume 1999 103 THE CHARACTERISTICS AND MANAGEMENT PROBLEMS OF THE VEGETATION AND FLORA OF THE HUNTINGFIELD AREA, SOUTHERN TASMANIA by J.B. Kirkpatrick (with two tables, four text-figures and one appendix) KIRKPATRICK, J.B., 1999 (31:x): The characteristics and management problems of the vegetation and flora of the Huntingfield area, southern Tasmania. Pap. Proc. R. Soc. Tasm. 133(1): 103-113. ISSN 0080-4703. School of Geography and Environmental Studies, University ofTasmania, GPO Box 252-78, Hobart, Tasmania, Australia 7001. The Huntingfield area has a varied vegetation, including substantial areas ofEucalyptus amygdalina heathy woodland, heath, buttongrass moorland and E. amygdalina shrubbyforest, with smaller areas ofwetland, grassland and E. ovata shrubbyforest. Six floristic communities are described for the area. Two hundred and one native vascular plant taxa, 26 moss species and ten liverworts are known from the area, which is particularly rich in orchids, two ofwhich are rare in Tasmania. Four other plant species are known to be rare and/or unreserved inTasmania. Sixty-four exotic plantspecies have been observed in the area, most ofwhich do not threaten the native biodiversity. However, a group offire-adapted shrubs are potentially serious invaders. Management problems in the area include the maintenance ofopen areas, weed invasion, pathogen invasion, introduced animals, fire, mechanised recreation, drainage from houses and roads, rubbish dumping and the gathering offirewood, sand and plants. Key Words: flora, forest, heath, Huntingfield, management, Tasmania, vegetation, wetland, woodland. INTRODUCTION species with the most cover in the shrub stratum (dominant species) was noted. If another species had more than half The Huntingfield Estate, approximately 400 ha of forest, the cover ofthe dominant one it was noted as a codominant. -

Rhizosphere Processes and Nutrient Management for Improving Nutrient
HORTSCIENCE 54(4):603–608. 2019. https://doi.org/10.21273/HORTSCI13643-18 macadamia production is still in its infancy. Many guide brochures on the Macadamia grower’s handbook have been used in Aus- Rhizosphere Processes and Nutrient tralia and America (Bittenbender and Hirae, 1990; O’Hare et al., 2004). The technical Management for Improving guidelines mentioned in these books are not well adapted to the local soil and climatic Nutrient-use Efficiency in conditions in China. Moreover, the unique characteristics of cluster roots of macadamia have been greatly ignored, leading to uncou- Macadamia Production pling of crop management in the orchard with Xin Zhao and Qianqian Dong root/rhizosphere-based nutrient management. Department of Plant Nutrition, China Agricultural University, Key Enhancing nutrient-use efficiency through op- timizing fertilizer input, improving fertilizer Laboratory of Plant–Soil Interactions, Ministry of Education, Beijing formulation, and maximizing biological in- 100193, P. R. China teraction effects helps develop healthy and sustainable orchards (Jiao et al., 2016; Shen Shubang Ni, Xiyong He, Hai Yue, and Liang Tao et al., 2013). Yunnan Institute of Tropical Crops, Jinghong 666100, Yunnan, P. R. China This paper discusses the problems and challenges of macadamia production and de- Yanli Nie velopment in China as well as other parts of The General Station of Forestry Technology Extension in Yunnan Province, the world, analyzes how cluster root growth Yunnan, P. R. China affects the rhizosphere dynamics of macad- amia, thus contributing to efficient nutrient Caixian Tang mobilization and use, and puts forward the Department of Animal, Plant and Soil Sciences, AgriBio – Centre for strategies of nutrient management for im- AgriBioscience, La Trobe University, Bundoora, Victoria 3086, Australia proving nutrient-use efficiency in sustainable macadamia production. -

Freschet Et Al., 2018), Sometimes Across Different Belowground Entities (Freschet & Roumet, 2017)
A starting guide to root ecology: strengthening ecological concepts and standardizing root classification, sampling, processing and trait measurements Gregoire Freschet, Loic Pagès, Colleen Iversen, Louise Comas, Boris Rewald, Catherine Roumet, Jitka Klimešová, Marcin Zadworny, Hendrik Poorter, Johannes Postma, et al. To cite this version: Gregoire Freschet, Loic Pagès, Colleen Iversen, Louise Comas, Boris Rewald, et al.. A starting guide to root ecology: strengthening ecological concepts and standardizing root classification, sampling, processing and trait measurements. 2020. hal-02918834 HAL Id: hal-02918834 https://hal.archives-ouvertes.fr/hal-02918834 Preprint submitted on 21 Aug 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. A starting guide to root ecology: strengthening ecological concepts and standardizing root classification, sampling, processing and trait measurements Grégoire T. Freschet1,2, Loïc Pagès3, Colleen M. Iversen4, Louise H. Comas5, Boris Rewald6, Catherine Roumet1, Jitka Klimešová7, Marcin Zadworny8, Hendrik Poorter9,10, Johannes A. Postma9, Thomas S. Adams11, Agnieszka Bagniewska-Zadworna12, A. Glyn Bengough13,14, Elison B. Blancaflor15, Ivano Brunner16, Johannes H.C. Cornelissen17, Eric Garnier1, Arthur Gessler18,19, Sarah E. Hobbie20, Ina C. Meier21, Liesje Mommer22, Catherine Picon-Cochard23, Laura Rose24, Peter Ryser25, Michael Scherer- Lorenzen26, Nadejda A. -

5 Priority Flora (Comesperma Rhadinocarpum, Desmocladus Elongatus, Hemiandra Sp
PO Box 437 Kalamunda WA 6926 +61 08 9257 1625 [email protected] (ACN 063 507 175, ABN 39 063 507 175) INTRODUCTION The following brief overview was prepared to assist in outlining some of the issues for planning rehabilitation needs on the proposed Silica sands operations by VRX Silica. As a summary of key points it raises some issues that need discussion with the operational team at VRX Silica. VEGETATION DIRECT TRANSFER (VDT) TRIAL A rehabilitation technique that was first implemented at Iluka Eneabba in 2012 by the Iluka site personnel. Since this time the Mattiske Consulting team have been assessing the progress of the plants from this method. As such it supplements the extensive rehabilitation practices on the Iluka operations which relies on the more classical approach of site preparation and seeding. Iluka differs from other rehabilitation areas on mining operations in that the native vegetation was also mulched and applied to the rehabilitation areas. The rehabilitation technique of VDT, or community translocation, is the practice of salvaging and replacing intact sods of vegetation with the underlying soil intact (Ross et al, 2000). The method trialled on the VDT 16 trial Vegetation Direct Transfer transects in 2019. The use of this technique by Iluka in the former Jennings mining area aims to improve the sustainability of translocating largely recalcitrant sedge and rush species, which tend to be less well represented in rehabilitation via other techniques, due to their low or complete lack of seed production (Norman and Koch 2007), but which often dominate local heath communities. The use of deep profile direct return of the topsoil and overburden in one pass may provide a large scale method of translocating rhizomatous and tuberous species in rehabilitated areas (Norman and Koch 2007). -

Isopogon Uncinatus)
Interim Recovery Plan No. 345 Albany Cone Bush (Isopogon uncinatus) Interim Recovery Plan 2014–2019 Department of Parks and Wildlife, Western Australia June 2014 Interim Recovery Plan for Isopogon uncinatus List of Acronyms The following acronyms are used in this plan: ADTFRT Albany District Threatened Flora Recovery Team BGPA Botanic Gardens and Parks Authority CALM Department of Conservation and Land Management CCWA Conservation Commission of Western Australia CITES Convention on International Trade in Endangered Species CR Critically Endangered DEC Department of Environment and Conservation DAA Department of Aboriginal Affairs DPaW Department of Parks and Wildlife (also shown as Parks and Wildlife and the department) DRF Declared Rare Flora EN Endangered EPBC Environment Protection and Biodiversity Conservation IBRA Interim Biogeographic Regionalisation for Australia IRP Interim Recovery Plan IUCN International Union for Conservation of Nature LGA Local Government Authority MRWA Main Roads Western Australia NRM Natural Resource Management PEC Priority Ecological Community RDL Department of Regional Development and Lands RP Recovery Plan SCB Species and Communities Branch SCD Science and Conservation Division SWALSC South West Aboriginal Land and Sea Council TEC Threatened Ecological Community TFSC Threatened Flora Seed Centre UNEP-WCMC United Nations Environment Program World Conservation Monitoring Centre VU Vulnerable WA Western Australia 2 Interim Recovery Plan for Isopogon uncinatus Foreword Interim Recovery Plans (IRPs) are developed within the framework laid down in Department of Parks and Wildlife Policy Statements Nos. 44 and 50 (CALM 1992; CALM 1994). Note: The Department of Conservation and Land Management (CALM) formally became the Department of Environment and Conservation (DEC) in July 2006 and the Department of Parks and Wildlife in July 2013. -

Edible Native Plants Cheeseberry Leptecophylla Juniperina Coast Beardheath Or Native Currant Coast Daisybush Olearia Axillaris Coastal Wattle Acacia Longifolia Subsp
Copperleaf Snowberry Gaultheria hispida Ants Delight Acrotriche serrulata Barilla or Grey Saltbush Atriplex cinerea Bidgee-widgee Acaena novae-zelandiae Bower Spinach Tetragonia implexicoma Cape Barren Tea Correa alba Copperleaf Snowberry Gaultheria hispida Running Postman Kennedia prostrata Woolly Teatree Leptospermum lanigerum Edible Native Plants Cheeseberry Leptecophylla juniperina Coast Beardheath or Native Currant Coast Daisybush Olearia axillaris Coastal Wattle Acacia longifolia subsp. sophorae Cranberry Heath Astroloma humifusum OF TASMANIA subsp. juniperina Yellow Everlastingbush Ozothamnus obcordatus Key PART OF PLANT USED Underground Leaves/Leaf Bases Flowers Fruit Part Creeping Strawberry Pine Cutting Grass Gahnia grandis Erect Currantbush Leptomeria drupacea Grasstree, yamina or Green Appleberry Billardiera mutabilis Microcachrys tetragona Geebung Persoonia spp. Yacca Xanthorrhoea australis Purple Appleberry Meristem/Bud Exudate/Sap Seeds PREPARATION AND USE Snack Process Cook Eat Raw Tea Sweet Drink Flavouring CAUTION Hazard / Toxin Harvest Kills Plant Heartberry Aristotelia peduncularis Kangaroo Apple Solanum laciniatum Leeklily Bulbine spp. Lemon-leaf Heathmyrtle Baeckea gunniana Macquarie Vine or Blue Flaxlily Dionella spp. River Mint Mentha australis Native Grape Muehlenbeckia spp. Manfern or lakri Dicksonia antarctica or Milkmaids Burchardia umbellata Mountain Pepper Tasmannia lanceolata Native Cherry Exocarpus cupressiformis Native Ivyleaf Violet Viola hederacea Native Raspberry Rubus pavifolius Cyathea ssp. Native Bluebell Wahlenbergia spp. More information Cautionary Notes This poster is only a guide to what’s potentially edible. - sance so be cautious. Consume any new or unfamiliar food in small quantities. Ensure fruits are fully ripe. Note it’s often best not to ingest seeds or pips. cultivation and contemporary use of our edible native plants is still an evolving art and science. Source plants for your garden from native plant nurseries. -

Pathogen Driven Change in Species-Diverse Woodlands of the Southwest Australian Floristic Region: a Hybrid Ecosystem in a Global Biodiversity Hotspot
Pathogen driven change in species-diverse woodlands of the Southwest Australian Floristic Region: A hybrid ecosystem in a Global Biodiversity Hotspot Carly Lauren Bishop A thesis submitted for the degree of Doctor of Philosophy at The University of Queensland in June 2012 School of Agriculture and Food Sciences ii Declaration by author This thesis is composed of my original work, and contains no material previously published or written by another person except where due reference has been made in the text. I have clearly stated the contribution by others to jointly-authored works that I have included in my thesis. I have clearly stated the contribution of others to my thesis as a whole, including statistical assistance, survey design, data analysis, significant technical procedures, professional editorial advice, and any other original research work used or reported in my thesis. The content of my thesis is the result of work I have carried out since the commencement of my research higher degree candidature and does not include a substantial part of work that has been submitted to qualify for the award of any other degree or diploma in any university or other tertiary institution. I have clearly stated which parts of my thesis, if any, have been submitted to qualify for another award. I acknowledge that an electronic copy of my thesis must be lodged with the University Library and, subject to the General Award Rules of The University of Queensland, immediately made available for research and study in accordance with the Copyright Act 1968. I acknowledge that copyright of all material contained in my thesis resides with the copyright holder(s) of that material.