Hierarchical Temporal Processing Deficit Model of Reality Distortion And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spatially Heterogeneous Choroid Plexus Transcriptomes Encode Positional Identity and Contribute to Regional CSF Production
The Journal of Neuroscience, March 25, 2015 • 35(12):4903–4916 • 4903 Development/Plasticity/Repair Spatially Heterogeneous Choroid Plexus Transcriptomes Encode Positional Identity and Contribute to Regional CSF Production Melody P. Lun,1,3 XMatthew B. Johnson,2 Kevin G. Broadbelt,1 Momoko Watanabe,4 Young-jin Kang,4 Kevin F. Chau,1 Mark W. Springel,1 Alexandra Malesz,1 Andre´ M.M. Sousa,5 XMihovil Pletikos,5 XTais Adelita,1,6 Monica L. Calicchio,1 Yong Zhang,7 Michael J. Holtzman,7 Hart G.W. Lidov,1 XNenad Sestan,5 Hanno Steen,1 XEdwin S. Monuki,4 and Maria K. Lehtinen1 1Department of Pathology, and 2Division of Genetics, Boston Children’s Hospital, Boston, Massachusetts 02115, 3Department of Pathology and Laboratory Medicine, Boston University School of Medicine, Boston, Massachusetts 02118, 4Department of Pathology and Laboratory Medicine, University of California Irvine School of Medicine, Irvine, California 92697, 5Department of Neurobiology and Kavli Institute for Neuroscience, Yale School of Medicine, New Haven, Connecticut 06510, 6Department of Biochemistry, Federal University of Sa˜o Paulo, Sa˜o Paulo 04039, Brazil, and 7Pulmonary and Critical Care Medicine, Department of Medicine, Washington University, St Louis, Missouri 63110 A sheet of choroid plexus epithelial cells extends into each cerebral ventricle and secretes signaling factors into the CSF. To evaluate whether differences in the CSF proteome across ventricles arise, in part, from regional differences in choroid plexus gene expression, we defined the transcriptome of lateral ventricle (telencephalic) versus fourth ventricle (hindbrain) choroid plexus. We find that positional identitiesofmouse,macaque,andhumanchoroidplexiderivefromgeneexpressiondomainsthatparalleltheiraxialtissuesoforigin.We thenshowthatmolecularheterogeneitybetweentelencephalicandhindbrainchoroidplexicontributestoregion-specific,age-dependent protein secretion in vitro. -

Disruption of the Neuronal PAS3 Gene in a Family Affected with Schizophrenia D Kamnasaran, W J Muir, M a Ferguson-Smith,Dwcox
325 ORIGINAL ARTICLE J Med Genet: first published as 10.1136/jmg.40.5.325 on 1 May 2003. Downloaded from Disruption of the neuronal PAS3 gene in a family affected with schizophrenia D Kamnasaran, W J Muir, M A Ferguson-Smith,DWCox ............................................................................................................................. J Med Genet 2003;40:325–332 Schizophrenia and its subtypes are part of a complex brain disorder with multiple postulated aetiolo- gies. There is evidence that this common disease is genetically heterogeneous, with many loci involved. See end of article for In this report, we describe a mother and daughter affected with schizophrenia, who are carriers of a authors’ affiliations t(9;14)(q34;q13) chromosome. By mapping on flow sorted aberrant chromosomes isolated from lym- ....................... phoblast cell lines, both subjects were found to have a translocation breakpoint junction between the Correspondence to: markers D14S730 and D14S70, a 683 kb interval on chromosome 14q13. This interval was found to Dr D W Cox, 8-39 Medical contain the neuronal PAS3 gene (NPAS3), by annotating the genomic sequence for ESTs and perform- Sciences Building, ing RACE and cDNA library screenings. The NPAS3 gene was characterised with respect to the University of Alberta, genomic structure, human expression profile, and protein cellular localisation to gain insight into gene Edmonton, Alberta T6G function. The translocation breakpoint junction lies within the third intron of NPAS3, resulting in the dis- 2H7, Canada; [email protected] ruption of the coding potential. The fact that the bHLH and PAS domains are disrupted from the remain- ing parts of the encoded protein suggests that the DNA binding and dimerisation functions of this Revised version received protein are destroyed. -

Transcription Factor P73 Regulates Th1 Differentiation
ARTICLE https://doi.org/10.1038/s41467-020-15172-5 OPEN Transcription factor p73 regulates Th1 differentiation Min Ren1, Majid Kazemian 1,4, Ming Zheng2, JianPing He3, Peng Li1, Jangsuk Oh1, Wei Liao1, Jessica Li1, ✉ Jonathan Rajaseelan1, Brian L. Kelsall 3, Gary Peltz 2 & Warren J. Leonard1 Inter-individual differences in T helper (Th) cell responses affect susceptibility to infectious, allergic and autoimmune diseases. To identify factors contributing to these response differ- 1234567890():,; ences, here we analyze in vitro differentiated Th1 cells from 16 inbred mouse strains. Haplotype-based computational genetic analysis indicates that the p53 family protein, p73, affects Th1 differentiation. In cells differentiated under Th1 conditions in vitro, p73 negatively regulates IFNγ production. p73 binds within, or upstream of, and modulates the expression of Th1 differentiation-related genes such as Ifng and Il12rb2. Furthermore, in mouse experimental autoimmune encephalitis, p73-deficient mice have increased IFNγ production and less dis- ease severity, whereas in an adoptive transfer model of inflammatory bowel disease, transfer of p73-deficient naïve CD4+ T cells increases Th1 responses and augments disease severity. Our results thus identify p73 as a negative regulator of the Th1 immune response, suggesting that p73 dysregulation may contribute to susceptibility to autoimmune disease. 1 Laboratory of Molecular Immunology and the Immunology Center, National Heart, Lung, and Blood Institute, Bethesda, MD 20892-1674, USA. 2 Department of Anesthesia, Stanford University School of Medicine, Stanford, CA 94305, USA. 3 Laboratory of Molecular Immunology, National Institute of Allergy and Infectious Diseases, Bethesda, MD 20892, USA. 4Present address: Department of Biochemistry and Computer Science, Purdue University, West ✉ Lafayette, IN 37906, USA. -

Regulation of Expression and Activity of the Bhlh-PAS Transcription
Regulation of Expression and Activity of the bHLH-PAS Transcription Factor NPAS4 David Christopher Bersten B.Sc. (Biomedical Science), Honours (Biochemistry) A thesis submitted in fulfilment of the requirements for the degree of Doctor of Philosophy Discipline of Biochemistry School of Molecular and Biomedical Science University of Adelaide, Australia June 2014 1 Contents Abstract ................................................................................................................................................... 3 PhD Thesis Declaration ........................................................................................................................... 5 Acknowledgements ................................................................................................................................. 6 Publications ............................................................................................................................................. 8 Conference oral presentations ........................................................................................................... 9 Additional publications ....................................................................................................................... 9 Chapter 1: .............................................................................................................................................. 10 Introduction ..................................................................................................................................... -

Steroid-Dependent Regulation of the Oviduct: a Cross-Species Transcriptomal Analysis
University of Kentucky UKnowledge Theses and Dissertations--Animal and Food Sciences Animal and Food Sciences 2015 Steroid-dependent regulation of the oviduct: A cross-species transcriptomal analysis Katheryn L. Cerny University of Kentucky, [email protected] Right click to open a feedback form in a new tab to let us know how this document benefits ou.y Recommended Citation Cerny, Katheryn L., "Steroid-dependent regulation of the oviduct: A cross-species transcriptomal analysis" (2015). Theses and Dissertations--Animal and Food Sciences. 49. https://uknowledge.uky.edu/animalsci_etds/49 This Doctoral Dissertation is brought to you for free and open access by the Animal and Food Sciences at UKnowledge. It has been accepted for inclusion in Theses and Dissertations--Animal and Food Sciences by an authorized administrator of UKnowledge. For more information, please contact [email protected]. STUDENT AGREEMENT: I represent that my thesis or dissertation and abstract are my original work. Proper attribution has been given to all outside sources. I understand that I am solely responsible for obtaining any needed copyright permissions. I have obtained needed written permission statement(s) from the owner(s) of each third-party copyrighted matter to be included in my work, allowing electronic distribution (if such use is not permitted by the fair use doctrine) which will be submitted to UKnowledge as Additional File. I hereby grant to The University of Kentucky and its agents the irrevocable, non-exclusive, and royalty-free license to archive and make accessible my work in whole or in part in all forms of media, now or hereafter known. -

A Simple Differentiation Protocol for Generation of Induced
1 Article, Special Issue "Induced Pluripotent Stem Cells in Neurodegenerative Diseases: Application for Therapy and 2 Disease Modeling" 3 A simple differentiation protocol for generation of 4 induced pluripotent stem cell-derived basal forebrain 5 cholinergic neurons for Alzheimer’s disease and 6 frontotemporal dementia disease modeling 7 Supplemental information 8 Method 1. Reprogramming and characterisation of MBE2960 healthy control iPSC line 9 The iPSCs were generated using skin fibroblasts obtained from subjects over the age of 18 years 10 by episomal method as described [40]. Briefly, reprogramming was performed on passage 8-10 11 fibroblasts by nucleofection (Lonza Amaxa Nucleofector) with episomal vectors expressing 12 OCT4, SOX2, KLF4, L-MYC, LIN28 and shRNA against p53 [41] in feeder- and serum- free 13 conditions using TeSR-E7 medium (Stemcell Technologies). Subsequently, reprogrammed 14 colonies were manually dissected to establish clonal cell lines [42]. Three clones were assessed 15 for pluripotency markers via immunocytochemistry (Figure S1A). The iPSC line was expanded 16 and characterised. Embryoid bodies were obtained as described [43] and using tri-lineage 17 differentiation kit (Stemcell Technologies). Germ layer differentiation was assessed by 18 immunochemistry (Figure S1B). Copy number variation (CNV) analysis of original fibroblasts 19 and iPSCs from MBE2960 (Figure S1C) was performed using Illumina HumanCore Beadchip 20 arrays as we described [40]. CNV analyses were performed using PennCNV and QuantiSNP with 21 default parameter settings [44,45]. Chromosomal aberrations were deemed to involve at least 10 22 contiguous single nucleotide polymorphisms (SNPs) or a genomic region spanning at least 1MB 23 [44,45]. The B allele frequency (BAF) and the log R ratio (LRR) were extracted from 24 GenomeStudio (Illumina) for representation (Figure S1D). -

Diagnosis of FOXG1 Syndrome Caused by Recurrent Balanced Chromosomal Rearrangements: Case Study and Literature Review Connor P
Craig et al. Mol Cytogenet (2020) 13:40 https://doi.org/10.1186/s13039-020-00506-1 CASE REPORT Open Access Diagnosis of FOXG1 syndrome caused by recurrent balanced chromosomal rearrangements: case study and literature review Connor P. Craig1,2, Emily Calamaro3, Chin‑To Fong3, Anwar M. Iqbal1, Alexander R. Paciorkowski3,4,5,6 and Bin Zhang1,3* Abstract Background: The FOXG1 gene plays a vital role in mammalian brain diferentiation and development. Intra‑ and intergenic mutations resulting in loss of function or altered expression of the FOXG1 gene cause FOXG1 syndrome. The hallmarks of this syndrome are severe developmental delay with absent verbal language, post‑natal growth restric‑ tion, post‑natal microcephaly, and a recognizable movement disorder characterized by chorea and dystonia. Case presentation: Here we describe a case of a 7‑year‑old male patient found to have a de novo balanced translo‑ cation between chromosome 3 at band 3q14.1 and chromosome 14 at band 14q12 via G‑banding chromosome and Fluorescence In Situ Hybridization (FISH) analyses. This rearrangement disrupts the proximity of FOXG1 to a previously described smallest region of deletion overlap (SRO), likely resulting in haploinsufciency. Conclusions: This case adds to the growing body of literature implicating chromosomal structural variants in the manifestation of this disorder and highlights the vital role of cis‑acting regulatory elements in the normal expression of this gene. Finally, we propose a protocol for refex FISH analysis to improve diagnostic efciency for patients with suspected FOXG1 syndrome. Keywords: FOXG1, Haploinsufciency, Postnatal microcephaly, FISH, Enhancer, Chromosomal rearrangement, Diagnosis Introduction brain development, with high levels of expression in the Te Forkhead Box G1 (FOXG1) gene [OMIM: 164874], developing fetal telencephalon [1–4]. -
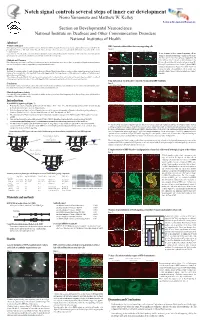
Notch Signal Controls Several Steps of Inner Ear Development Norio Yamamoto and Matthew W
NIDCD Notch signal controls several steps of inner ear development Norio Yamamoto and Matthew W. Kelley Section on Developmental Neuroscience Section on Developmental Neuroscience National Institute on Deafness and Other Communication Disorders National Institutes of Health Abstract Problem addressed RBP-J mutant cochleae did not have any supporting cells Notch signaling has been reported to contribute to inner ear development, however, its specific functions remain unclear, partly because of discrepancies between the phenotypes of mutant mice with single deletion of specific Notch related genes. These discrepancies are probably due to functional compensation by other Notch Figure 4 receptors or ligands. Foxg1 Cre;RBP-J floxed/+ Foxg1 Cre;RBP-J floxed/floxed A B C D To determine the effects of complete elimination of Notch signaling, we used a conditional knockout of the Rbpsuh gene. RBP-J protein is a critical transcriptional p27 Phalloidin p27 Phalloidin To test if mutant cochleae contained supporting cells we co-activator for all Notch molecules and thus deletion of this protein inhibits all Notch signaling. examined expression of supporting cell markers such as p27 and Prox1. On E17.5 p27 was expressed in supporting cells Methods and Measures ***** ***** under or between hair cells such as inner pharyngeal cells, Floxed Rbpsuh mice were crossed with Foxg1-Cre knock-in mice to delete the Rbpsuh gene in the inner ear. Inner ear phenotypes in Rbpsuh conditional knockout Deiter's cells and pillar cells (asterisks in figure 4 A and B). mice were determined at various developmental stages using immunohistochemistry. But no p27 expression was detected in those regions of RBP-J E Prox1 F Phalloidin G Prox1 H Phalloidin conditional knockout mice (Figure 4 C and D). -

Emx2 Is Required for Growth of the Hippocampus but Not for Hippocampal Field Specification
The Journal of Neuroscience, April 1, 2000, 20(7):2618–2625 Emx2 Is Required for Growth of the Hippocampus But Not for Hippocampal Field Specification Shubha Tole,1 Guy Goudreau,2 Stavroula Assimacopoulos,1 and Elizabeth A. Grove1 1Department of Neurobiology, Pharmacology, and Physiology, University of Chicago, Chicago, Illinois 60637, and 2Max Planck Institute of Biophysical Chemistry, D-37077 Goettingen, Germany The vertebrate Emx genes are expressed in a nested pattern in positioned in the Emx2 mutant. In particular, a dentate cell early embryonic cerebral cortex, such that a medial strip of population is generated, although it fails to form a morpholog- cortex expresses Emx2 but not Emx1. This pattern suggests ical gyrus. This failure may be part of a more widespread medial that Emx genes could play a role in specifying different areas or cortical defect in the mutant. Examination of cortical cell pro- fields of the cortex along the mediolateral axis. Such a role has liferation and differentiation indicates a disruption of the matu- been supported by the observation that in mice lacking func- ration of the medial cortex in the absence of Emx2. Thus, Emx2 tional Emx2 the hippocampus is shrunken and the most medial is required for normal growth and maturation of the hippocam- field of the cortex, the hippocampal dentate gyrus, appears by pus but not for the specification of cells to particular hippocam- cytoarchitecture to be missing (Pellegrini et al., 1996; Yoshida et pal field identities. al., 1997). Use of region-specific molecular markers shows, Key words: Emx2; hippocampus; patterning; specification; however, that hippocampal fields are specified and correctly cortical maturation; cortical hem The hippocampus, like the rest of the cerebral cortex, is divided Reports that specific mutations lead to morphological defects in into cytoarchitectonic areas or fields (Nauta and Feirtag, 1986). -

Downloaded from Bioscientifica.Com at 09/26/2021 08:38:14PM Via Free Access
1 185 L C Gregory, P Gergics, OTX2 mutations in congenital 185:1 121–135 Clinical Study M Nakaguma and others hypopituitarism The phenotypic spectrum associated with OTX2 mutations in humans Louise C Gregory 1,*, Peter Gergics2,* , Marilena Nakaguma3,* , Hironori Bando2 , Giuseppa Patti1,4,5, Mark J McCabe1, Qing Fang 2, Qianyi Ma2 , Ayse Bilge Ozel2 , Jun Z Li2 , Michele Moreira Poina3, Alexander A L Jorge 3, Anna F Figueredo Benedetti3, Antonio M Lerario3, Ivo J P Arnhold3 , Berenice B Mendonca3 , Mohamad Maghnie4,5, Sally A Camper2 , Luciani R S Carvalho3 and Mehul T Dattani1 1Section of Molecular Basis of Rare Disease, Genetics and Genomic Medicine Research & Teaching Department, UCL Great Ormond Street Institute of Child Health, London, UK, 2Department of Human Genetics, University of Michigan, Correspondence Ann Arbor, Michigan, USA, 3Developmental Endocrinology Unit, Hospital das Clinicas da Faculdade de Medicina da should be addressed Universidade de São Paulo, São Paulo, Brazil, 4Department of Pediatrics, IRCCS Istituto Giannina Gaslini and to S A Camper or M T 5Department of Neuroscience, Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health, University of Dattani Genova, Genova, Italy Email *(L C Gregory, P Gergics and M Nakaguma contributed equally to this work) [email protected] or [email protected] Abstract Objective: The transcription factor OTX2 is implicated in ocular, craniofacial, and pituitary development. Design: We aimed to establish the contribution of OTX2 mutations in congenital hypopituitarism patients with/without eye abnormalities, study functional consequences, and establish OTX2 expression in the human brain, with a view to investigate the mechanism of action. Methods: We screened patients from the UK (n = 103), international centres (n = 24), and Brazil (n = 282); 145 were within the septo-optic dysplasia spectrum, and 264 had no eye phenotype. -
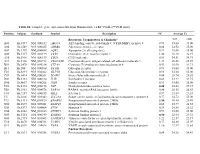
Table S1. Complete Gene Expression Data from Human Diabetes RT² Profiler™ PCR Array Receptors, Transporters & Channels* A
Table S1. Complete gene expression data from Human Diabetes RT² Profiler™ PCR Array Position Unigene GenBank Symbol Description FC Average Ct Receptors, Transporters & Channels* NGT GDM A01 Hs,5447 NM_000352 ABCC8 ATP-binding cassette, sub-family C (CFTR/MRP), member 8 0.93 35.00 35.00 A04 0Hs,2549 NM_000025 ADRB3 Adrenergic, beta-3-, receptor 0.88 34.92 35.00 A07 Hs,1307 NM_000486 AQP2 Aquaporin 2 (collecting duct) 0.93 35.00 35.00 A09 30Hs,5117 NM_001123 CCR2 Chemokine (C-C motif) receptor 2 1.00 26.28 26.17 A10 94Hs,5916 396NM_006139 CD28 CD28 molecule 0.81 34.51 34.71 A11 29Hs,5126 NM_001712 CEACAM1 Carcinoembryonic antigen-related cell adhesion molecule 1 1.31 26.08 25.59 B01 82Hs,2478 NM_005214 CTLA4 (biliaryCytotoxic glycoprotein) T-lymphocyte -associated protein 4 0.53 30.90 31.71 B11 24Hs,208 NM_000160 GCGR Glucagon receptor 0.93 35.00 35.00 C01 Hs,3891 NM_002062 GLP1R Glucagon-like peptide 1 receptor 0.93 35.00 35.00 C07 03Hs,6434 NM_000201 ICAM1 Intercellular adhesion molecule 1 0.84 28.74 28.89 D02 47Hs,5134 NM_000418 IL4R Interleukin 4 receptor 0.64 34.22 34.75 D06 57Hs,4657 NM_000208 INSR Insulin receptor 0.93 35.00 35.00 E05 44Hs,4312 NM_006178 NSF N-ethylmaleimide-sensitive factor 0.48 28.42 29.37 F08 79Hs,2961 NM_004578 RAB4A RAB4A, member RAS oncogene family 0.88 20.55 20.63 F10 69Hs,7287 NM_000655 SELL Selectin L 0.97 23.89 23.83 F11 56Hs,3806 NM_001042 SLC2A4 Solute carrier family 2 (facilitated glucose transporter), member 4 0.77 34.72 35.00 F12 91Hs,5111 NM_003825 SNAP23 Synaptosomal-associated protein, 23kDa 3.90 -

The Role of Emx1 and Emx2 in the Developing Chick Telencephalon
The Role of Emx1 and Emx2 in the developing chick telencephalon Dissertation Zur Erlangung des Doktorgrades der Naturwissenschaften (Dr.rer.nat.) der Fakultät für Biologie der Ludwig-Maximilian-Universität München Angefertigt am Max-Planck-Institut für Neurobiologie in der Arbeitsgruppe Neuronale Spezifizierung und in der GSF am Institut für Stammzellforschung Julia von Frowein München, Dezember 2004 1. Gutachter: Prof.Dr. Magdalena Götz 2. Gutachter: Prof.Dr. George Boyan eingereicht am 20.12.2004 Tag der mündlichen Prüfung: 26.4.2005 If the brain were so simple we could understand it, we would be so simple we couldn't. Lyall Watson Table of content 1 Table of content 1 Table of content...........................................................................................1 2 Abstract ........................................................................................................5 3 Zusammenfassung......................................................................................6 4 Introduction..................................................................................................8 4.1 General development of the regions of the central nervous system ....................................8 4.2 Patterning and regionalization .............................................................................................8 4.3 Regions of the forebrain.....................................................................................................10 4.4 Migration............................................................................................................................12