UNIVERSITY of CALIFORNIA, SAN DIEGO the Role
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Snapper and Grouper: SFP Fisheries Sustainability Overview 2015
Snapper and Grouper: SFP Fisheries Sustainability Overview 2015 Snapper and Grouper: SFP Fisheries Sustainability Overview 2015 Snapper and Grouper: SFP Fisheries Sustainability Overview 2015 Patrícia Amorim | Fishery Analyst, Systems Division | [email protected] Megan Westmeyer | Fishery Analyst, Strategy Communications and Analyze Division | [email protected] CITATION Amorim, P. and M. Westmeyer. 2016. Snapper and Grouper: SFP Fisheries Sustainability Overview 2015. Sustainable Fisheries Partnership Foundation. 18 pp. Available from www.fishsource.com. PHOTO CREDITS left: Image courtesy of Pedro Veiga (Pedro Veiga Photography) right: Image courtesy of Pedro Veiga (Pedro Veiga Photography) © Sustainable Fisheries Partnership February 2016 KEYWORDS Developing countries, FAO, fisheries, grouper, improvements, seafood sector, small-scale fisheries, snapper, sustainability www.sustainablefish.org i Snapper and Grouper: SFP Fisheries Sustainability Overview 2015 EXECUTIVE SUMMARY The goal of this report is to provide a brief overview of the current status and trends of the snapper and grouper seafood sector, as well as to identify the main gaps of knowledge and highlight areas where improvements are critical to ensure long-term sustainability. Snapper and grouper are important fishery resources with great commercial value for exporters to major international markets. The fisheries also support the livelihoods and food security of many local, small-scale fishing communities worldwide. It is therefore all the more critical that management of these fisheries improves, thus ensuring this important resource will remain available to provide both food and income. Landings of snapper and grouper have been steadily increasing: in the 1950s, total landings were about 50,000 tonnes, but they had grown to more than 612,000 tonnes by 2013. -

Using Molecular Identification of Ichthyoplankton to Monitor
Molecular Identification of Ichthyoplankton in Cabo Pulmo National Park 1 Using molecular identification of ichthyoplankton to monitor 2 spawning activity in a subtropical no-take Marine Reserve 3 4 5 6 Ana Luisa M. Ahern1, *, Ronald S. Burton1, Ricardo J. Saldierna-Martínez2, Andrew F. Johnson1, 7 Alice E. Harada1, Brad Erisman1,4, Octavio Aburto-Oropeza1, David I. Castro Arvizú3, Arturo R. 8 Sánchez-Uvera2, Jaime Gómez-Gutiérrez2 9 10 11 12 1Marine Biology Research Division, Scripps Institution of Oceanography, University of California 13 San Diego, La Jolla, California, USA 14 2Departamento de Plancton y Ecología Marina, Centro Interdisciplinario de Ciencias Marinas, 15 Instituto Politécnico Nacional, CP 23096, La Paz, Baja California Sur, Mexico 16 3Cabo Pulmo National Park, Baja California Sur, Mexico 17 4The University of Texas at Austin, Marine Science Institute, College of Natural Sciences, 18 Port Aransas, Texas, USA 19 20 21 22 23 24 25 *Corresponding author: [email protected] 1 Molecular Identification of Ichthyoplankton in Cabo Pulmo National Park 26 ABSTRACT: Ichthyoplankton studies can provide valuable information on the species richness 27 and spawning activity of fishes, complementing estimations done using trawls and diver surveys. 28 Zooplankton samples were collected weekly between January and December 2014 in Cabo 29 Pulmo National Park, Gulf of California, Mexico (n=48). Fish larvae and particularly eggs are 30 difficult to identify morphologically, therefore the DNA barcoding method was employed to 31 identify 4,388 specimens, resulting in 157 Operational Taxonomic Units (OTUs) corresponding 32 to species. Scarus sp., Halichoeres dispilus, Xyrichtys mundiceps, Euthynnus lineatus, 33 Ammodytoides gilli, Synodus lacertinus, Etrumeus acuminatus, Chanos chanos, Haemulon 34 flaviguttatum, and Vinciguerria lucetia were the most abundant and frequent species recorded. -

Alternative Formats If You Require This Document in an Alternative Format, Please Contact: [email protected]
Citation for published version: Cox, JPL 2008, 'Hydrodynamic aspects of fish olfaction', Journal of The Royal Society Interface, vol. 5, no. 23, pp. 575-593. https://doi.org/10.1098/rsif.2007.1281 DOI: 10.1098/rsif.2007.1281 Publication date: 2008 Document Version Peer reviewed version Link to publication The definitive version is available as: Cox, J. P. L., 2008. Hydrodynamic aspects of fish olfaction. Journal of The Royal Society Interface, 5 (23), pp. 575-593. DOI: http://dx.doi.org/10.1098/rsif.2007.1281 University of Bath Alternative formats If you require this document in an alternative format, please contact: [email protected] General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Download date: 04. Oct. 2021 HYDRODYNAMIC ASPECTS OF FISH OLFACTION Jonathan P. L. Cox Department of Chemistry, University of Bath, Bath BA2 7AY, UK [email protected] Tel. +44 1 225 386548 Fax +44 1 225 386231 1 SUMMARY Flow into and around the olfactory chamber of a fish determines how odorant from the fish’s immediate environment is transported to the sensory surface (olfactory epithelium) lining the chamber. Diffusion times in water are long, even over comparatively short distances (millimetres). -
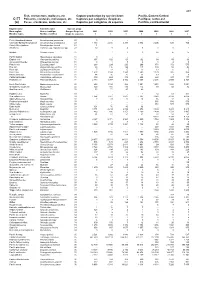
Fish, Crustaceans, Molluscs, Etc Capture Production by Species
489 Fish, crustaceans, molluscs, etc Capture production by species items Pacific, Eastern Central C-77 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Pacifique, centre-est (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Pacífico, centro-oriental English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 2001 2002 2003 2004 2005 2006 2007 Nombre inglés Nombre científico Grupo de especies t t t t t t t Pink(=Humpback)salmon Oncorhynchus gorbuscha 23 0 - - - - - - Chinook(=Spring=King)salmon Oncorhynchus tshawytscha 23 1 193 2 416 3 278 2 992 2 205 538 724 Coho(=Silver)salmon Oncorhynchus kisutch 23 1 - - - - - - Smelts nei Osmerus spp, Hypomesus spp 23 12 8 4 5 1 0 1 Milkfish Chanos chanos 25 ... 1 2 3 14 15 15 Pacific halibut Hippoglossus stenolepis 31 0 - - - - - - English sole Pleuronectes vetulus 31 197 102 63 102 69 59 66 Arrow-tooth flounder Atheresthes stomias 31 0 1 5 2 3 2 3 Petrale sole Eopsetta jordani 31 268 224 220 266 371 372 575 Rex sole Glyptocephalus zachirus 31 98 116 124 117 109 54 42 Rock sole Lepidopsetta bilineata 31 7 13 15 13 13 6 5 Dover sole Microstomus pacificus 31 1 403 1 994 1 943 1 590 1 179 826 864 Pacific sand sole Psettichthys melanostictus 31 44 35 27 22 19 7 6 California flounder Paralichthys californicus 31 389 424 379 456 422 327 177 Flatfishes nei Pleuronectiformes 31 2 383 1 977 2 442 2 367 2 236 2 599 2 324 North Pacific hake Merluccius productus 32 853 1 194 988 1 091 1 651 1 683 1 651 Grenadiers, rattails nei Macrouridae 32 145 156 130 114 99 58 82 Gadiformes nei Gadiformes 32 53 71 47 40 - - - Hagfishes nei Myxinidae 33 - - - 1 54 68 302 Sea catfishes nei Ariidae 33 1 964 2 161 1 097 1 287 3 948 4 174 4 056 Squirrelfishes nei Holocentridae 33 0 24 22 18 15 12 16 Flathead grey mullet Mugil cephalus 33 .. -

ASFIS ISSCAAP Fish List February 2007 Sorted on Scientific Name
ASFIS ISSCAAP Fish List Sorted on Scientific Name February 2007 Scientific name English Name French name Spanish Name Code Abalistes stellaris (Bloch & Schneider 1801) Starry triggerfish AJS Abbottina rivularis (Basilewsky 1855) Chinese false gudgeon ABB Ablabys binotatus (Peters 1855) Redskinfish ABW Ablennes hians (Valenciennes 1846) Flat needlefish Orphie plate Agujón sable BAF Aborichthys elongatus Hora 1921 ABE Abralia andamanika Goodrich 1898 BLK Abralia veranyi (Rüppell 1844) Verany's enope squid Encornet de Verany Enoploluria de Verany BLJ Abraliopsis pfefferi (Verany 1837) Pfeffer's enope squid Encornet de Pfeffer Enoploluria de Pfeffer BJF Abramis brama (Linnaeus 1758) Freshwater bream Brème d'eau douce Brema común FBM Abramis spp Freshwater breams nei Brèmes d'eau douce nca Bremas nep FBR Abramites eques (Steindachner 1878) ABQ Abudefduf luridus (Cuvier 1830) Canary damsel AUU Abudefduf saxatilis (Linnaeus 1758) Sergeant-major ABU Abyssobrotula galatheae Nielsen 1977 OAG Abyssocottus elochini Taliev 1955 AEZ Abythites lepidogenys (Smith & Radcliffe 1913) AHD Acanella spp Branched bamboo coral KQL Acanthacaris caeca (A. Milne Edwards 1881) Atlantic deep-sea lobster Langoustine arganelle Cigala de fondo NTK Acanthacaris tenuimana Bate 1888 Prickly deep-sea lobster Langoustine spinuleuse Cigala raspa NHI Acanthalburnus microlepis (De Filippi 1861) Blackbrow bleak AHL Acanthaphritis barbata (Okamura & Kishida 1963) NHT Acantharchus pomotis (Baird 1855) Mud sunfish AKP Acanthaxius caespitosa (Squires 1979) Deepwater mud lobster Langouste -

Petition to Ban Vaquita-Harming Fish and Fish Products From
May 18, 2017 PETITION TO BAN IMPORTS OF FISH AND FISH PRODUCTS FROM MEXICO THAT RESULT IN THE INCIDENTAL KILL OR SERIOUS INJURY OF VAQUITA IN EXCESS OF UNITED STATES STANDARDS PURSUANT TO MARINE MAMMAL PROTECTION ACT SECTION 101 BEFORE THE DEPARTMENT OF HOMELAND SECURITY, THE DEPARTMENT OF THE TREASURY, AND THE DEPARTMENT OF COMMERCE Natural Resources Defense Council Center for Biological Diversity Animal Welfare Institute NOTICE OF PETITION John Kelly, Secretary Steven Mnuchin, Secretary Department of Homeland Security Department of the Treasury Washington, DC 20528 1500 Pennsylvania Ave., NW Washington, DC 20220 Wilbur Ross, Secretary Kevin McAleenan, Acting Commissioner Department of Commerce Customs and Border Protection 1401 Constitution Ave., NW Department of Homeland Security Washington, DC 20230 1300 Pennsylvania Ave., NW Washington, DC 20229 Sam Rauch Acting Assistant Administrator National Marine Fisheries Service National Oceanographic and Atmospheric Administration 1315 East-West Highway Silver Spring, MD 20910 Petitioners Natural Resources Defense Council Center for Biological Diversity 1314 2nd St. 2400 NW 80th St., #146 Santa Monica, CA 90401 Seattle, WA 98117 Tel: (310) 434-2300 Tel: (206) 327-2344 Animal Welfare Institute 900 Pennsylvania Ave., SE Washington, DC 20003 Tel: (202) 337-2332 The Natural Resources Defense Council (“NRDC”) is an international nonprofit environmental organization with more than 2 million members and online activists. Since 1970, our lawyers, scientists, and other environmental specialists have worked to protect the world's natural resources, public health, and the environment. NRDC has offices in New York City, Washington, D.C., Los Angeles, San Francisco, Chicago, Bozeman, and Beijing. NRDC and its members are concerned with the conservation of marine mammals and the effective implementation of the Marine Mammal Protection Act. -

Fish, Crustaceans, Molluscs, Etc Capture Production by Species
491 Fish, crustaceans, molluscs, etc Capture production by species items Pacific, Eastern Central C-77 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Pacifique, centre-est (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Pacífico, centro-oriental English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 2002 2003 2004 2005 2006 2007 2008 Nombre inglés Nombre científico Grupo de especies t t t t t t t Chinook(=Spring=King)salmon Oncorhynchus tshawytscha 23 2 416 3 278 2 992 2 205 538 724 - Smelts nei Osmerus spp, Hypomesus spp 23 8 4 5 1 0 1 0 Milkfish Chanos chanos 25 1 2 3 14 15 15 15 English sole Pleuronectes vetulus 31 102 63 102 69 59 66 72 Arrow-tooth flounder Atheresthes stomias 31 1 5 2 3 2 3 1 Petrale sole Eopsetta jordani 31 224 220 266 371 372 575 549 Rex sole Glyptocephalus zachirus 31 116 124 117 109 54 42 38 Rock sole Lepidopsetta bilineata 31 13 15 13 13 6 5 2 Dover sole Microstomus pacificus 31 1 994 1 943 1 590 1 179 826 864 1 046 Pacific sand sole Psettichthys melanostictus 31 35 27 22 19 7 6 3 California flounder Paralichthys californicus 31 424 379 456 422 327 177 214 Flatfishes nei Pleuronectiformes 31 1 977 2 442 2 367 2 227 2 251 3 052 2 988 North Pacific hake Merluccius productus 32 1 194 988 1 091 1 651 1 683 1 651 1 650 Grenadiers, rattails nei Macrouridae 32 156 130 114 99 58 82 85 Gadiformes nei Gadiformes 32 71 47 40 - - - - Hagfishes nei Myxinidae 33 - - 1 54 68 302 416 Sea catfishes nei Ariidae 33 2 161 1 097 1 287 3 948 4 174 2 813 2 635 Squirrelfishes nei Holocentridae 33 24 22 18 15 12 16 27 Flathead grey mullet Mugil cephalus 33 .. -

Family-Lutjanidae-Overview-PDF-Update.Pdf
FAMILY Lutjanidae Gill 1861 - snappers [=Acanthopomes, Mesopriontes, Aphareoidei, Lutjaninae, Hoplopagrinae, Platyiniini, Etelinae, Aprioninae, Paradicichthyinae, Symphorinae, Neomaenidae, Apsilinae] GENUS Aphareus Cuvier, in Cuvier & Valeniennes, 1830 - snappers, jobfish [=Fares, Humefordia, Playtpodus, Sacrestinus, Ulapiscis] Species Aphareus furca (Lacepede, 1801) - smalltoothed jobfish [=caerulescens, elegans, flavivultus, pikea, sacrestinus] Species Aphareus rutilans Cuvier, in Cuvier & Valenciennes, 1830 - rusty jobfish [=thompsoni] GENUS Aprion Valenciennes, in Cuvier & Valenciennes, 1830 - snappers, jobfish [=Sparopsis] Species Aprion virescens Valenciennes, in Cuvier & Valenciennes, 1830 - green jobfish [=elongatus, kanekonis, latifrons, microchir, placidus] GENUS Apsilus Valenciennes, in Cuvier & Valenciennes, 1830 - snappers [=Tropidinius] Species Apsilus dentatus Guichenot, 1853 - black snapper [=arnillo] Species Apsilus fuscus Valenciennes, in Cuvier & Valenciennes, 1830 - African forktail snapper GENUS Etelis Cuvier, 1828 - snappers [=Elastoma, Erythrobussothen, Etelinus, Hesperanthias, Macrops] Species Etelis carbunculus Cuvier, in Cuvier & Valenciennes, 1828 - deepwater red snapper [=marshi] Species Etelis coruscans Valenciennes, 1862 - deepwater longtail red snapper [=evurus, lifuensis] Species Etelis oculatus (Valenciennes, 1828) - queen snapper [=gracilis] Species Etelis radiosus Anderson, 1981 - pale snapper GENUS Hoplopagrus Gill, 1861 - snapper [=Deuteracanthus] Species Hoplopagrus guentherii Gill, 1862 - Mexican -

Coastal Fisheries of Latin America and the Caribbean
FAO ISSN 2070-7010 FISHERIES AND AQUACULTURE TECHNICAL PAPER 544 Coastal fisheries of Latin America and the Caribbean Cover photos and credits (from top left clockwise): Fishing boat with bottom nets for hoki in Tierra del Fuego, Argentina (courtesy of Miguel S. Isla); landing products in Holbox, Quintana Roo, Mexico (courtesy of Mizue Oe); artisanal boat operating in Santa Marta, Colombia (courtesy of Mario Rueda); artisanal fisher fishing octopus in Yucatán, Mexico (courtesy of Manuel Solis); lobster boat with traps in Cuba (Centro de Investigaciones Pesqueras de Cuba); artisanal boat operating in Santa Marta, Colombia (courtesy of Mario Rueda). FAO FISHERIES AND Coastal fisheries of AQUACULTURE TECHNICAL Latin America and PAPER the Caribbean 544 Edited by Silvia Salas Centro de Investigación y de Estudios Avanzados Unidad Mérida Mérida, Yucatán, Mexico Ratana Chuenpagdee Memorial University of Newfoundland St. John’s, Newfoundland, Canada Anthony Charles Saint Mary’s University Halifax, Nova Scotia, Canada Juan Carlos Seijo Universidad Marista de Mérida Mérida, Yucatán, Mexico FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 2011 The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its [ whether or not these have been patterned, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned. -

Pre-Assessment Report of the Marine Finfish Fishery Caught with Handlines in Guaymas, Sonora, Mexico
Pre-assessment report of the marine finfish fishery caught with handlines in Guaymas, Sonora, Mexico Prepared for: S.C.P.P. 29 de agosto. S.C.P.P. El Resbalón. S.C.P.P. El Mirador de la Manga. S.C.P.P. Pesquera La Manga Restaurante Doña Rosita. C.P.P. Pesquera Los Sazanes. S.C.P.P. Francisco Flores. S.C.P.P. Alianza de Pescadores de Guaymas. S.C.P.P. Las Dallanas. S.C.P.P. Pescadores de la Cantera. Prepared by: Comunidad y Biodiversidad, A. C. Francisco Javier Fernández Rivera Melo, Alesa Flores Guzmán & José Francisco Chávez Reviewed by Mónica Valle-Esquivel, Jodi Bostrom, Erin Wilson Validated by MRAG Americas April 2020 Report by Comunidad y Biodiversidad, AC Validated by (CAB) MRAG Americas Inc. Sociedad Cooperativa de Producción Pesquera 29 de agosto. Sociedad Cooperativa de Producción Pesquera El Resbalón. Sociedad Cooperativa de Producción Pesquera El Mirador de la Manga. Sociedad Cooperativa de Producción Pesquera La Manga Restaurante Doña Rosita. Fishery client Cooperativa de Producción Pesquera Los Sazanes. Sociedad Cooperativa de Producción Pesquera Francisco Flores. Sociedad Cooperativa de Producción Pesquera Alianza de Pescadores de Guaymas. Sociedad Cooperativa de Producción Pesquera Las Dallanas. Sociedad Cooperativa de Producción Pesquera Pescadores de la Cantera. Assessment Type Comprehensive pre-assessment Project code US2647_COBI_MX_PA_Validation Version 3.0 Francisco Javier Fernández Rivera Melo, Alesa Flores Guzmán & Prepared by Jose Francisco Chavez Mónica Valle-Esquivel, Jodi Bostrom, Erin Wilson (MRAG Reviewed by Americas) Approved by Amanda Stern-Pirlot Comunidad y Biodiversidad, A. C. Calle Isla del Peruano #215 Colonia Lomas de Miramar Guaymas, Sonora, México, CP 85448 Correo electrónico [email protected] (información) 2 1 Contents 1 Contents .................................................................................................................... -

Marine Ecoregions of North America
Marine ecoregions o f n o rth a m e r i c a Marine ecoregions o f n o r t h a m e r i c a This report was written for the Secretariat of the Commission for Environmental Cooperation as a collaborative effort with the following authors of the Marine Ecoregions Project Team. Its publication has been made possible thanks to the generous support of its copublishers. Numerous individuals have contributed to the completion of this work (please see the acknowledgments section for additional information). The information contained herein is the responsibility of the authors and does not necessarily reflect the views of the CEC, or the governments of Canada, Mexico or the United States of America. Authors Tara A. C. Wilkinson, McGill University, Commission for Environmental Cooperation (CEC) Ed Wiken, Wildlife Habitat Canada (WHC) Juan Bezaury Creel, The Nature Conservancy Mexico (TNC Mexico) Thomas F. Hourigan, National Oceanic and Atmospheric Administration (NOAA) Tundi Agardy, Sound Seas Hans Herrmann, Commission for Environmental Cooperation (CEC) Lisa Janishevski, Commission for Environmental Cooperation (CEC) Chris Madden, Natureserve Lance Morgan, Marine Conservation Biology Institute (MCBI) Moreno Padilla, Canadian Council on Ecological Areas (CCEA) Co-Publishers Comisión Nacional de Áreas Naturales Protegidas Comisión Nacional para el Conocimiento y Uso de la Biodiversidad National Oceanic and Atmospheric Administration Parks Canada Instituto Nacional de Ecología Reproduction of this publication, in whole or in part and in whatever form, may be done without seeking express authorization of the CEC, provided that the reproduced material is to be used for educational or nonprofit purposes and that it cites the source of the original. -

Ecological Sustainability of Small-Scale Fisheries the Case of a Seafood Supply Chain in Baja California Sur, Mexico
! ! !!Stockholm!Resilience!Centre! !!!!Research!for!Biosphere!Stewardship!and!Innovation! ! Master’s Thesis, 60 ECTS! Social-ecological Resilience for Sustainable Development Master’s programme 2015/17, 120 ECTS How traders and their institutional arrangements influence the social- ecological sustainability of small-scale fisheries The case of a seafood supply chain in Baja California Sur, Mexico ! ! ! ! ! ! !! ! ! !! ! ! Blanca González García-Mon The uncertain air that magnified some things and blotted out others hung over the whole Gulf so that all sights were unreal and vision could not be trusted: so that sea and land had the sharp clarities and the vagueness of a dream. (…) There was no certainty in seeing, no proof that what you saw was there or was not there. And the people of the Gulf expected all places were that way, and it was not strange to them. The Pearl, John Steinbeck ! ABSTRACT' The production of Small-Scale Fisheries (SSFs) satisfies seafood demand and is an important contributor to people’s livelihoods around the world. The disconnection between supply and demand can influence sustainability of SSFs by decreasing actors’ ability to respond to environmental and market changes. In developing contexts, such as many Mexican fishing communities, fishers and traders (fish buyers) establish self-governing instructional arrangements that connect local fish production with demand across scales. This thesis studies the mediating role of traders’ institutional arrangements for the social-ecological (SES) sustainability of a supply chain. A mixed-method approach is applied to a study case in Baja California Sur, Mexico, that includes interviews, surveys, participant observation and a multi-level network analysis.