Chemical Inventory
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Industry Compliance Programme
Global Chemical Industry Compliance Programme GC-ICP Chemical Weapons Convention December 2006 Version 1.0 GLOBAL CHEMICAL INDUSTRY COMPLIANCE PROGRAMME FOR IMPLEMENTING THE CHEMICAL WEAPONS CONVENTION The purpose of the handbook is to provide guidance to chemical facilities, traders and trading companies in developing a Global Chemical Industry Compliance Programme (GC-ICP) to comply with the Chemical Weapons Convention (CWC). The GC-ICP focuses first on determining if there is a reporting requirement to your National Authority and second on collecting the relevant support data used to complete the required reports. The GC-ICP is designed to provide a methodology to comply with the CWC and establish systems that facilitate and demonstrate such compliance. Each facility/company should also ensure that it follows its country’s CWC specific laws, regulations and reporting requirements. • Sections 2, 3, and 4 guide you through the process of determining if chemicals at your facility/ company should be reported to your National Authority for compliance with the CWC. • Section 5 provides recommended guidance on information that you may use to determine your reporting requirements under the CWC and administrative tools that your facility/company may use to ensure compliance with the CWC. • Section 6 provides a glossary of terms and associated acronyms. • Section 7 provides a listing of all National Authorities by country. CWC Global Chemical Industry Compliance Programme 1 TABLE OF CONTENTS Section 1 Overview What is the Chemical Weapons Convention? -

Copyrighted Material
1 Historical Milieu 1.1 Organophosphorus Nerve Agents 2 1.2 Blister Agents 5 1.3 Sternutator Agents 11 1.4 Chemical Weapons Convention (CWC) 13 1.4.1 Schedule of Chemicals 14 1.4.2 Destruction of Chemical Weapons 14 References 16 COPYRIGHTED MATERIAL Analysis of Chemical Warfare Degradation Products, First Edition. Karolin K. Kroening, Renee N. Easter, Douglas D. Richardson, Stuart A. Willison and Joseph A. Caruso. © 2011 John Wiley & Sons, Ltd. Published 2011 by John Wiley & Sons, Ltd. 2 ANALYSIS OF CHEMICAL WARFARE DEGRADATION PRODUCTS 1.1 ORGANOPHOSPHORUS NERVE AGENTS Organophosphorus (OP) type compounds, that is, deriva- tives containing the P=O moiety, were first discovered in the 1800s when researchers were investigating useful applica- tions for insecticides/rodenticides. There are many derivatives of organophosphorus compounds, however, the OP deriva- tives that are typically known as ‘nerve agents’ were discov- ered accidentally in Germany in 1936 by a research team led by Dr. Gerhard Schrader at IG Farben [1–4]. Schrader had noticed the effects and lethality of these organophosphorus compounds towards insects and began developing a new class of insecticides. While working towards the goal of an improved insecticide, Schrader experimented with numerous phosphorus-containing compounds, leading to the discovery of the first nerve agent, Tabun (or GA) (Figure 1.1). The potency of these insecticides towards humans was not realized until there was yet another accident, which involved a Tabun spill. Schrader and coworkers began experiencing symptoms, such as miosis (constriction of the pupils of the eyes), dizziness and severe shortness of breath, with numerous effects lasting several weeks [1, 4, 5]. -

Cancer Drug Pharmacology Table
CANCER DRUG PHARMACOLOGY TABLE Cytotoxic Chemotherapy Drugs are classified according to the BC Cancer Drug Manual Monographs, unless otherwise specified (see asterisks). Subclassifications are in brackets where applicable. Alkylating Agents have reactive groups (usually alkyl) that attach to Antimetabolites are structural analogues of naturally occurring molecules DNA or RNA, leading to interruption in synthesis of DNA, RNA, or required for DNA and RNA synthesis. When substituted for the natural body proteins. substances, they disrupt DNA and RNA synthesis. bendamustine (nitrogen mustard) azacitidine (pyrimidine analogue) busulfan (alkyl sulfonate) capecitabine (pyrimidine analogue) carboplatin (platinum) cladribine (adenosine analogue) carmustine (nitrosurea) cytarabine (pyrimidine analogue) chlorambucil (nitrogen mustard) fludarabine (purine analogue) cisplatin (platinum) fluorouracil (pyrimidine analogue) cyclophosphamide (nitrogen mustard) gemcitabine (pyrimidine analogue) dacarbazine (triazine) mercaptopurine (purine analogue) estramustine (nitrogen mustard with 17-beta-estradiol) methotrexate (folate analogue) hydroxyurea pralatrexate (folate analogue) ifosfamide (nitrogen mustard) pemetrexed (folate analogue) lomustine (nitrosurea) pentostatin (purine analogue) mechlorethamine (nitrogen mustard) raltitrexed (folate analogue) melphalan (nitrogen mustard) thioguanine (purine analogue) oxaliplatin (platinum) trifluridine-tipiracil (pyrimidine analogue/thymidine phosphorylase procarbazine (triazine) inhibitor) -

Safe Methods of Use 14: HSNO Class 6.1 Acutely Toxic Compounds
Safe Methods of Use 14: HSNO Class 6.1 Acutely Toxic Compounds Purpose: This applies to principal investigators (PIs), sector managers, designated laboratory person (DLPs), technical staff and students who use laboratories within the University of Auckland. Note: the word ‘shall denotes a mandatory requirement and the word ‘should’ denotes a recommendation. HSNO Class 6.1 Toxic Compounds will cover a wide range of chemicals, for which an exhaustive list cannot be supplied. Always consult MSDS sheets prior to handling any chemical, observe precautions and follow the recommendations for their handling. The mandatory recommendations in the SMOU will apply to HSNO Class 6.1 A and B compounds and should be treated as recommendations for handling HSNO Class 6.1C compounds where this appropriate. MSDS databases (Gold FFX) is available via the LEARN Database Appendix 1 provides a list (albeit not exhaustive) of HSNO 6.1 A, B and C Acutely Toxic Compounds with their classifications for your guidance. Please also note that chemicals that have primary HSNO classification of Class 3, 4, 5 or 8 may also be toxic. A. Incompatibilities Care should be taken to keep HSNO Class 6.1A and B compounds well away from liquid acids, bases, strongly oxidising solutions and reactive compounds. Safe Methods of Use 14 – HSNO Class 6.1 Acute Toxics Version 7 Feb 2019 Page 1 of 9 B. Storage 1. Containers with toxic compounds with an oral LD50 less than 5 milligrams/kg (HSNO 6.1A), shall be clearly labelled with identity of compound and a warning indicating their toxicity. 2. -

Supplement to All Hazards Receipt Facility (AHRF) Screening Protocol
EPA/600/R-10/155 December 2010 Supplement to All Hazards Receipt Facility (AHRF) Screening Protocol SCIENCE Office of Research and Development National Homeland Security Research Center Supplement to All Hazards Receipt Facility (AHRF) Screening Protocol December 2010 Office of Research and Development National Homeland Security Research Center Acknowledgments This document is intended to be supplementary to the U.S. Environmental Protection Agency (EPA) and U.S. Department of Homeland Security (DHS) September 2008 All Hazards Receipt Facility Protocol (AHRF Protocol), and attempts to address considerations raised by stakeholders since publication of the protocol. Development of this document was funded by the U.S. Environmental Protection Agency (EPA) National Homeland Security Research Center (NHSRC), and includes information provided by EPA Regions 1, 6, and 10; EPA Office of Radiation and Indoor Air (ORIA), the Association of Public Health Laboratories (APHL): State Public Health Laboratories of Connecticut, Delaware, Massachusetts, Minnesota, New Jersey, New York, and Virginia; and New York City; and the Canadian Defence Research and Development Laboratory. This document was prepared by CSC under Contract EP-W-06- 046. Disclaimer This document is intended to be supplementary to the guidance provided in the U.S. Environmental Protection Agency (EPA) and U.S. Department of Homeland Security (DHS) September 2008 All Hazards Receipt Facility Protocol (AHRF Protocol), and attempts to address considerations raised by stakeholders since publication of the protocol. This supplement assumes that: • The September 2008 AHRF Protocol was developed and provided as a guide; implementation of the protocol and the screening equipment included in the protocol may vary among locations, depending on the goals and capabilities of the laboratory to which the facility is attached. -

Graphical Arrays of Chemical-Specific Health Effect Reference Values for Inhalation Exposures
EPA/600/R-09/061 Graphical Arrays of Chemical-Specific Health Effect Reference Values for Inhalation Exposures Includes Errata Sheet created on 4/6/2010 September 2009 U.S. Environmental Protection Agency Office of Research and Development National Center for Environmental Assessment Research Triangle Park, NC DISCLAIMER This document has been prepared by staff from the Hazardous Pollutant Assessment Group, National Center for Environmental Assessment, U.S. Environmental Protection Agency. Any opinions, findings, conclusions, or recommendations are those of the authors and do not necessarily reflect the views of the EPA. For questions concerning this document, please contact Dr. George Woodall (919-541-3896; [email protected]). September 2009 ii Errata Sheet Created 4/6/2010 for the document titled Graphical Arrays of Chemical-Specific Health Effect Reference Values for Inhalation Exposures, Final Table or Page Erratum Figure 133 Changed “values” to “value,” deleted “and OSHA,” and added “OSHA PEL” before “ACGIH ” in the first sentence of the second paragraph. Added the following sentence at the end of the second paragraph: “It should also be noted that the original documentaion for the OSHA PEL cited it as a Ceiling Value (OSHA, 1996, 192249) but OSHA later clarified in a memo that the value was a time-weighted average (OSHA, 1996, 598129)” Figure 133 Replaced Figure 2.15 2.15 Table 137 Replaced “OSHA-Ceiling” with “OSHA-PEL (TWA)” in the first 2.15 column of Table 2.15. Replaced “10 min” with “8 hr TWA” in the second column. Added reference in last column. 140 Added reference “OSHA (1996). -

The Effect of High-Dose Thiotepa, Alone Or in Combination with Other
Bone Marrow Transplantation (2007) 40, 891–896 & 2007 Nature Publishing Group All rights reserved 0268-3369/07 $30.00 www.nature.com/bmt ORIGINAL ARTICLE The effect of high-dose thiotepa, alone or in combination with other chemotherapeutic agents, on a murine B-cell leukemia model simulating autologous stem cell transplantation A Abdul-Hai, L Weiss, D Ergas, IB Resnick, S Slavin and MY Shapira Department of Bone Marrow Transplantation and Cancer Immunotherapy, Hadassah–Hebrew University Medical Center, Jerusalem, Israel The use of thiotepa (TH) is increasing, especially in stem Introduction cell transplantation, mainly due to its safety and blood– brain barrier penetration. We evaluated the use of TH in a Thiotepa (TH, triethylene thiophosphoramide), an ethylene murine model simulating autologous stem cell transplan- amide, developed by Lederle Laboratories in 1952, tation, with or without additional agents. Between 1 and possesses mechlorethamine-like alkylating activity and 11 days following inoculation of BALB/c mice with 105– has been used clinically for over 35 years.1 It is of particular 108 B-cell leukemia (BCL1) cells (simulating pre-trans- use in breast cancer, mainly as second-line treatment.2 plant leukemia loads), each group received an ‘induction- TH has been given intrathecally for carcinomatous like’ irradiation and/or cytotoxic regimen. Animals were meningitis, and intravesically for bladder carcinoma.3,4 either followed without treatment, or an adoptive transfer Mild-to-moderate activity was observed in several solid (AT) was performed to untreated BALB/c mice. Admi- tumors and hematological malignancies.1,5 There has nistered alone without AT, high-dose TH did not change been, however, a sustained interest in defining clinical roles the time to appearance of leukemia. -

SUMMARY of PARTICULARLY HAZARDOUS SUBSTANCES (By
SUMMARY OF PARTICULARLY HAZARDOUS SUBSTANCES (by alpha) Key: SC -- Select Carcinogens RT -- Reproductive Toxins AT -- Acute Toxins SA -- Readily Absorbed Through the Skin DHS -- Chemicals of Interest Revised: 11/2012 ________________________________________________________ ___________ _ _ _ _ _ _ _ _ _ _ _ ||| | | | CHEMICAL NAME CAS # |SC|RT| AT | SA |DHS| ________________________________________________________ ___________ | _ | _ | _ | _ | __ | | | | | | | 2,4,5-T 000093-76-5 | | x | | x | | ABRIN 001393-62-0 | | | x | | | ACETALDEHYDE 000075-07-0 | x | | | | | ACETAMIDE 000060-35-5 | x | | | | | ACETOHYDROXAMIC ACID 000546-88-3 ||x| | x | | ACETONE CYANOHYDRIN, STABILIZED 000075-86-5 | | | x | | x | ACETYLAMINOFLUORENE,2- 000053-96-3 | x | | | | | ACID MIST, STRONG INORGANIC 000000-00-0 | x | | | | | ACROLEIN 000107-02-8 | | x | x | x | | ACRYLAMIDE 000079-06-1 | x | x | | x | | ACRYLONITRILE 000107-13-1 | x | x | x | x | | ACTINOMYCIN D 000050-76-0 ||x| | x | | ADIPONITRILE 000111-69-3 | | | x | | | ADRIAMYCIN 023214-92-8 | x | | | | | AFLATOXIN B1 001162-65-8 | x | | | | | AFLATOXIN M1 006795-23-9 | x | | | | | AFLATOXINS 001402-68-2 | x | | x | | | ALL-TRANS RETINOIC ACID 000302-79-4 | | x | | x | | ALPRAZOMAN 028981-97-7 | | x | | x | | ALUMINUM PHOSPHIDE 020859-73-8 | | | x | | x | AMANTADINE HYDROCHLORIDE 000665-66-7 | | x | | x | | AMINO-2,4-DIBROMOANTHRAQUINONE 000081-49-2 | x | | | | | AMINO-2-METHYLANTHRAQUINONE, 1- 000082-28-0 | x | | | | | AMINO-3,4-DIMETHYL-3h-IMIDAZO(4,5f)QUINOLINE,2- 077094-11-2 | x | | | | | AMINO-3,8-DIMETHYL-3H-IMIDAZO(4,5-f)QUINOXALINE, -

Selected Analytical Methods for Environmental Remediation and Recovery (SAM) 2017
EPA/600/R-17/356 | September 2017 www.epa.gov/homeland-security-research Selected Analytical Methods for Environmental Remediation and Recovery (SAM) 2017 Office of Research and Development Homeland Security Research Program This page left intentionally blank EPA/600/R-17/356 | September 2017 Selected Analytical Methods for Environmental Remediation and Recovery (SAM) 2017 UNITED STATES ENVIRONMENTAL PROTECTION AGENCY Cincinnati, OH 45268 Office of Research and Development Homeland Security Research Program Disclaimer Disclaimer The U.S. Environmental Protection Agency (EPA) through its Office of Research and Development funded and managed the research described here under Contract EP-C-15-012 to CSRA Inc. This document is undergoing review and has not been approved for publication. The contents reflect the views of the contributors and technical work groups and do not necessarily reflect the views of the Agency. Mention of trade names or commercial products in this document or in the methods referenced in this document does not constitute endorsement or recommendation for use. Questions concerning this document or its application should be addressed to: Romy Campisano National Homeland Security Research Center Office of Research and Development (NG16) U.S. Environmental Protection Agency 26 West Martin Luther King Drive Cincinnati, OH 45268 (513) 569-7016 [email protected] Kathy Hall National Homeland Security Research Center Office of Research and Development (NG16) U.S. Environmental Protection Agency 26 West Martin Luther King -
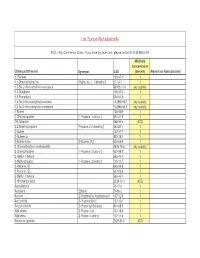
Homeland Security List
List Sorted Alphabetically ACG = Any Commercial Grade. If you have any questions, please contact EHS @ 898-5126. Minimum Concentration Chemical Of Interest Synonym CAS (percent) Amount on Hand (pounds) 1- Pentene 109-67-1 1 1,1-Dimethylhydrazine [Hydrazine, 1, 1-dimethyl-] 57-14-7 1 1,3-Bis(2-chloroethylthio)-n-propane 63905-10-2 any quantity 1,3-Butadiene 106-99-0 1 1,3-Pentadiene 504-60-9 1 1,4-Bis(2-chloroethylthio)-n-butane 142868-93-7 any quantity 1,5-Bis(2-chloroethylthio)-n-pentane 142868-94-8 any quantity 1-Butene 106-98-9 1 1-Chloropropylene [1-Propene, 1-chloro-] 590-21-6 1 1H-Tetrazole 288-94-8 ACG 2,2-Dimethylpropane [Propane, 2,2-dimethyl-] 463-82-1 1 2-Butene 107-01-7 1 2-Butene-cis 590-18-1 1 2-Butene-trans [2-Butene, (E)] 624-64-6 1 2-Chloroethylchloro-methylsulfide 2625-76-5 any quantity 2-Chloropropylene [1-Propene, 2-chloro-] 557-98-2 1 2-Methyl-1-butene 563-46-2 1 2-Methylpropene [1-Propene, 2-methyl-] 115-11-7 1 2-Pentene, (E)- 646-04-8 1 2-Pentene, (Z)- 627-20-3 1 3-Methyl-1-butene 563-45-1 1 5-Nitrobenzotriazol 2338-12-7 ACG Acetaldehyde 75-07-0 1 Acetylene [Ethyne] 74-86-2 1 Acrolein [2-Propenal] or Acrylaldehyde 107-02-8 1 Acrylonitrile [2-Propenenitrile] 107-13-1 1 Acrylyl chloride [2-Propenoyl chloride] 814-68-6 1 Allyl alcohol [2-Propen-1-ol] 107-18-6 1 Allylamine [2-Propen-1-amine] 107-11-9 1 Aluminum (powder) 7429-90-5 ACG Ammonia (anhydrous) 7664-41-7 1 Ammonia (conc. -

Things to Be Done
DRAFT MAY 2003 ANNEX 1: CHEMICAL AGENTS 1. Introduction The large-scale use of toxic chemicals as weapons first became possible during the First World War (1914–1918) thanks to the growth of the chemical industry. More than 110 000 tonnes were disseminated over the battlefields, the greater part on the western front. Initially, the chemicals were used, not to cause casualties in the sense of putting enemy combatants out of action, but rather to harass. Though the sensory irritants used were powerful enough to disable those who were exposed to them, they served mainly to drive enemy combatants out of the trenches or other cover that protected them from conventional fire, or to disrupt enemy artillery or supplies. About 10% of the total tonnage of chemical warfare agents used during the war were chemicals of this type, namely lacrimators (tear gases), sternutators and vomiting agents. However, use of more lethal chemicals soon followed the introduction of disabling chemicals. In all, chemical agents caused some 1.3 million casualties, including 90 000 deaths. During the First World War, almost every known noxious chemical was screened for its potential as a weapon, and this process was repeated during the Second World War (1939–1945), when substantial stocks of chemical weapons were accumulated, although rarely used in military operations. Between the two world wars, a high proportion of all the new compounds that had been synthesized, or isolated from natural materials, were examined to determine their utility as lethal or disabling chemical weapons. After 1945, these systematic surveys continued, together with a search for novel agents based on advances in biochemistry, toxicology and pharmacology. -

Provisional Peer-Reviewed Toxicity Values for Lewisite (Casrn 541-25-3)
EPA/690/R-15/009F l Final 9-30-2015 Provisional Peer-Reviewed Toxicity Values for Lewisite (CASRN 541-25-3) Superfund Health Risk Technical Support Center National Center for Environmental Assessment Office of Research and Development U.S. Environmental Protection Agency Cincinnati, OH 45268 AUTHORS, CONTRIBUTORS, AND REVIEWERS CHEMICAL MANAGER Dan D. Petersen, PhD, DABT National Center for Environmental Assessment, Cincinnati, OH DRAFT DOCUMENT PREPARED BY SRC, Inc. 7502 Round Pond Road North Syracuse, NY 13212 PRIMARY INTERNAL REVIEWERS Jeffery Swartout, PhD, DABT National Center for Environmental Assessment, Cincinnati, OH This document was externally peer reviewed under contract to: Eastern Research Group, Inc. 110 Hartwell Avenue Lexington, MA 02421 3136 Questions regarding the contents of this document may be directed to the U.S. EPA Office of Research and Development’s National Center for Environmental Assessment, Superfund Health Risk Technical Support Center (513-569-7300). ii Lewisite TABLE OF CONTENTS COMMONLY USED ABBREVIATIONS AND ACRONYMS .................................................. iv BACKGROUND .............................................................................................................................1 DISCLAIMERS ...............................................................................................................................1 QUESTIONS REGARDING PPRTVs ............................................................................................1 INTRODUCTION ...........................................................................................................................2