Contamination by Hazardous Substances in the Gulf Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Rough Guide to Naples & the Amalfi Coast
HEK=> =K?:;I J>;HEK=>=K?:;je CVeaZh i]Z6bVaÒ8dVhi D7FB;IJ>;7C7B<?9E7IJ 7ZcZkZcid BdcYgV\dcZ 8{ejV HVc<^dg\^d 8VhZgiV HVciÉ6\ViV YZaHVcc^d YZ^<di^ HVciVBVg^V 8{ejVKiZgZ 8VhiZaKdaijgcd 8VhVaY^ Eg^cX^eZ 6g^Zcod / AV\dY^EVig^V BVg^\a^Vcd 6kZaa^cd 9WfeZ_Y^_de CdaV 8jbV CVeaZh AV\dY^;jhVgd Edoojda^ BiKZhjk^jh BZgXVidHVcHZkZg^cd EgX^YV :gXdaVcd Fecf[__ >hX]^V EdbeZ^ >hX]^V IdggZ6ccjco^ViV 8VhiZaaVbbVgZY^HiVW^V 7Vnd[CVeaZh GVkZaad HdggZcid Edh^iVcd HVaZgcd 6bVa[^ 8{eg^ <ja[d[HVaZgcd 6cVX{eg^ 8{eg^ CVeaZh I]Z8Vbe^;aZ\gZ^ Hdji]d[CVeaZh I]Z6bVa[^8dVhi I]Z^haVcYh LN Cdgi]d[CVeaZh FW[ijkc About this book Rough Guides are designed to be good to read and easy to use. The book is divided into the following sections, and you should be able to find whatever you need in one of them. The introductory colour section is designed to give you a feel for Naples and the Amalfi Coast, suggesting when to go and what not to miss, and includes a full list of contents. Then comes basics, for pre-departure information and other practicalities. The guide chapters cover the region in depth, each starting with a highlights panel, introduction and a map to help you plan your route. Contexts fills you in on history, books and film while individual colour sections introduce Neapolitan cuisine and performance. Language gives you an extensive menu reader and enough Italian to get by. 9 781843 537144 ISBN 978-1-84353-714-4 The book concludes with all the small print, including details of how to send in updates and corrections, and a comprehensive index. -

Rankings Municipality of Gaeta
9/24/2021 Maps, analysis and statistics about the resident population Demographic balance, population and familiy trends, age classes and average age, civil status and foreigners Skip Navigation Links ITALIA / Lazio / Province of Latina / Gaeta Powered by Page 1 L'azienda Contatti Login Urbistat on Linkedin Adminstat logo DEMOGRAPHY ECONOMY RANKINGS SEARCH ITALIA Municipalities Powered by Page 2 Aprilia Stroll up beside >> L'azienda Contatti Login Urbistat on Linkedin Lenola AdminstatBassiano logo DEMOGRAPHY ECONOMY RANKINGS SEARCH Maenza Campodimele ITALIA Minturno Castelforte Monte San Cisterna di Biagio Latina Norma Cori Pontinia Fondi Ponza Formia Priverno Gaeta Prossedi Itri Rocca Massima Latina Roccagorga Roccasecca dei Volsci Sabaudia San Felice Circeo Santi Cosma e Damiano Sermoneta Sezze Sonnino Sperlonga Spigno Saturnia Terracina Ventotene Provinces FROSINONE RIETI LATINA ROMA VITERBO Regions Powered by Page 3 Abruzzo Liguria L'azienda Contatti Login Urbistat on Linkedin AdminstatBasilicata logo Lombardia DEMOGRAPHY ECONOMY RANKINGS SEARCH Calabria MarcheITALIA Campania Molise Città del Piemonte Vaticano Puglia Emilia-Romagna Repubblica di Friuli-Venezia San Marino Giulia Sardegna Lazio Sicilia Toscana Trentino-Alto Adige/Südtirol Umbria Valle d'Aosta/Vallée d'Aoste Veneto Municipality of Gaeta Territorial extension of Municipality of GAETA and related population density, population per gender and number of households, average age and incidence of foreigners TERRITORY DEMOGRAPHIC DATA (YEAR 2019) Region Lazio Province Latina Inhabitants (N.) 20,071 Sign Province LT Families (N.) 9,217 Hamlet of the Males (%) 48.0 0 municipality Females (%) 52.0 Surface (Km2) 29.20 Foreigners (%) 4.4 Population density 687.3 Average age (Inhabitants/Kmq) 47.9 (years) Powered by Page 4 Average annual L'azienda Contatti Login Urbistat on Linkedin variation -0.84 Adminstat logo (2014/2019) DEMOGRAPHY ECONOMY RANKINGS SEARCH ITALIA MALES, FEMALES AND DEMOGRAPHIC BALANCE FOREIGNERS INCIDENCE (YEAR 2019) (YEAR 2019) Balance of nature [1], Migrat. -

Central and Southern Italy Campania, Molise, Abruzzo, Marche, Umbria and Lazio Garigliano
EUROPEAN COMMISSION DIRECTORATE-GENERAL FOR ENERGY DIRECTORATE D - Nuclear Safety and Fuel Cycle Radiation Protection Main Conclusions of the Commission’s Article 35 verification NATIONAL MONITORING NETWORK FOR ENVIRONMENTAL RADIOACTIVITY Central and Southern Italy Campania, Molise, Abruzzo, Marche, Umbria and Lazio DISCHARGE AND ENVIRONMENTAL MONITORING Garigliano NPP Date: 12 to 17 September 2011 Verification team: Mr C. Gitzinger (team leader) Mr E. Henrich Mr. E. Hrnecek Mr. A. Ryan Reference: IT-11/06 INTRODUCTION Article 35 of the Euratom Treaty requires that each Member State shall establish facilities necessary to carry out continuous monitoring of the levels of radioactivity in air, water and soil and to ensure compliance with the basic safety standards (1). Article 35 also gives the European Commission (EC) the right of access to such facilities in order that it may verify their operation and efficiency. For the EC, the Directorate-General for Energy (DG ENER) and in particular its Radiation Protection Unit (at the time of the visit ENER.D.4, now ENER.D.3) is responsible for undertaking these verifications. The main purpose of verifications performed under Article 35 of the Euratom Treaty is to provide an independent assessment of the adequacy of monitoring facilities for: - Liquid and airborne discharges of radioactivity into the environment by a site (and control thereof). - Levels of environmental radioactivity at the site perimeter and in the marine, terrestrial and aquatic environment around the site, for all relevant pathways. - Levels of environmental radioactivity on the territory of the Member State. Taking into account previous bilateral protocols, a Commission Communication has been published in the Official Journal on 4 July 2006 with a view to define some practical arrangements for the conduct of Article 35 verification visits in Member States. -
CAPRI È...A Place of Dream. (Pdf 2,4
Posizione geografica Geographical position · Geografische Lage · Posición geográfica · Situation géographique Fra i paralleli 40°30’40” e 40°30’48”N. Fra i meridiani 14°11’54” e 14°16’19” Est di Greenwich Superficie Capri: ettari 400 Anacapri: ettari 636 Altezza massima è m. 589 Capri Giro dell’isola … 9 miglia Clima Clima temperato tipicamente mediterraneo con inverno mite e piovoso ed estate asciutta Between parallels Zwischen den Entre los paralelos Entre les paralleles 40°30’40” and 40°30’48” Breitenkreisen 40°30’40” 40°30’40”N 40°30’40” et 40°30’48” N N and meridians und 40°30’48” N Entre los meridianos Entre les méridiens 14°11’54” and 14°16’19” E Zwischen den 14°11’54” y 14°16’19” 14e11‘54” et 14e16’19“ Area Längenkreisen 14°11’54” Este de Greenwich Est de Greenwich Capri: 988 acres und 14°16’19” östl. von Superficie Surface Anacapri: 1572 acres Greenwich Capri: 400 hectáreas; Capri: 400 ha Maximum height Fläche Anacapri: 636 hectáreas Anacapri: 636 ha 1,920 feet Capri: 400 Hektar Altura máxima Hauter maximum Distances in sea miles Anacapri: 636 Hektar 589 m. m. 589 from: Naples 17; Sorrento Gesamtoberfläche Distancias en millas Distances en milles 7,7; Castellammare 13; 1036 Hektar Höchste marinas marins Amalfi 17,5; Salerno 25; Erhebung über den Nápoles 17; Sorrento 7,7; Naples 17; Sorrento 7,7; lschia 16; Positano 11 Meeresspiegel: 589 Castellammare 13; Castellammare 13; Amalfi Distance round the Entfernung der einzelnen Amalfi 17,5; Salerno 25; 17,5 Salerno 25; lschia A Place of Dream island Orte von Capri, in Ischia 16; Positano 11 16; Positano 11 9 miles Seemeilen ausgedrükt: Vuelta a la isla por mar Tour de l’ île par mer Climate Neapel 17, Sorrento 7,7; 9 millas 9 milles Typical moderate Castellammare 13; Amalfi Clima Climat Mediterranean climate 17,5; Salerno 25; lschia templado típicamente Climat tempéré with mild and rainy 16; Positano 11 mediterráneo con typiquement Regione Campania Assessorato al Turismo e ai Beni Culturali winters and dry summers. -

Cry Havoc Règles Fr 05/01/14 17:46 Page1 Guiscarduiscard
maquette historique UK v2_cry havoc règles fr 05/01/14 17:46 Page1 Guiscarduiscard HISTORY & SCENARIOS maquette historique UK v2_cry havoc règles fr 05/01/14 17:46 Page2 © Buxeria & Historic’One éditions - 2014 - v1.1 maquette historique UK v2_cry havoc règles fr 05/01/14 17:46 Page1 History Normans in Southern Italy and Sicily in the 11th Century 1 - The historical context 1.1 - Southern Italy and Sicily at the beginning of the 11th Century Byzantium had conquered Southern Italy and Sicily in the first half of the 6th century. But by the end of that century, Lombards coming from Northern Italy had conquered most of the peninsula, with Byzantium retaining only Calabria and Sicily. From the middle of the 9th century, the Aghlabid Dynasty of Ifrîquya (the original name of Eastern Maghreb) raided Sicily to take possession of the island. A new Byzantine offensive at the end of the century took back most of the lost territories in Apulia and Calabria and established Bari as the new provincial capital. Lombard territories further north were broken down between three cities led by princes: Capua, Salerno, and Benevento. Further east, Italian duchies of Naples, Amalfi, and Gaeta tried to keep their autonomy through successive alliances with the various regional powers to try and maintain their commercial interests. Ethnic struggles in Sicily between Arabs and Berbers on the one side, and various dynasties on the other side, led to power fragmentation: The island is divided between four rival military factions at the beginning of the 11th century. Beyond its natural boundaries, Southern Italy had to cope with two external powers which were looking to expel Byzantium from what they considered was part of their area of influence: the Papacy and the Holy Roman Empire. -

AD Default.Pdf
our tenter de comprendre l'engouement qu'a toujours suscité Capri, perle de la Méditerranée, il faut s'y rendre à bord de l'un des ferries qui, tous les vendredis après-midi, de mai à septembre, quittent Naples, depuis le môle Beverello, en direction de l'île. Accou- dés au bastingage, les hommes bavardent gaiement sur leur téléphone portable der- nier cri avec leurs amis moins chanceux, retenus au travail à Naples, à Rome ou à Milan. Les femmes - de la grande bour- geoisie ou de l'aristocratie italiennes - affi- chent un look qui rappelle celui de la Jackie Kennedy des années 50: pantalon en coton style pêcheur, sandales à talon plat ou espadrilles à semelle de corde et haut pastel. Seules concessions à l'esprit du temps: le sac à main Gucci ou Prada et les lunettes de soleil Versace ou Fendi. Quelques bijoux de corail ou de turquoises soulignent le caractère décidément estival de cette tenue de villégiature. L'engouement des élites pour Capri n'est pas chose nouvelle. Déjà l'empereur Tibère décidait, en l'an 27 de notre ère, d'aban- donner Rome pour venir s'y installer... Dès le tournant du XVIIe siècle, l'île enchanta 1. Le grand salon de la villa Fersen est orné de marbres précieux, colonnes, stucs, et dorures. 2. Les fêtes organisées parle baron Jacques d'Adelsward -Fersen s'achevaient dans la chambre à opium. les «étrangers». D'abord des Allemands, léopard et se rendait à dîner chez ses amis des Français, des Anglais et des Suédois, avec un serpent enroulé au poignet en puis des Russes et enfin des Américains qui guise de bracelet.. -

Elenco Scuole Disponibili Ad Accogliere Le Gare Regionali - CAMPANIA
Elenco scuole disponibili ad accogliere le gare regionali - CAMPANIA Candidata Candidata polo reg Città Scuola polo reg Secondo Primo Ciclo Ciclo Scisciano Amodeo bethoven EBOLI I.C. ROMANO X CALVIZZANO I.C. MARCO POLO Mignano Monte Lungo I.C. Mignano M.L.- Marzano Bacoli I.C. Plinio il vecchio - Gramsci X X Bacoli I.C. Plinio il vecchio - Gramsci X Atripalda I.C. 'De Amicis - Masi' Vairano Patenora I.C. 'Garibaldi- Montalcini' I.C. 'Ilaria Alpi' - Scuola Secondaria di Primo Grado MONTESARCHIO X X 'Ugo Foscolo' Scisciano I.C. 'Omodeo - Beethoven' X Scisciano I.C. 'Omodeo - Beethoven' X Scisciano I.C. 'Omodeo - Beethoven' X Scisciano I.C.'Omodeo-Beethoven'- plesso centrale- Scisciano I.C.'Omodeo-Beethoven'- plesso succursale- San San Vitaliano Vitaliano Sant'Anastasia I.C:'F.d'Assisi-N.Amore' Benevento I.I.S. GALILEI VETRONE X Sant'Agata de' Goti I.I.S. 'A.M. de' Liguori' NAPOLI IC 2 Moscati Maglione NAPOLI IC 2 Moscati Maglione MONTECORVINO PUGLIANO IC MONTECORVINO PUGLIANO X MONTECORVINO PUGLIANO IC MONTECORVINO PUGLIANO X MONTECORVINO PUGLIANO (SA) IC Montecorvino Pugliano MONTECORVINO PUGLIANO (SA) IC Montecorvino Pugliano CASALNUOVO DI NAPOLI ICS 'ENRICO DE NICOLA' X CAPACCIO PAESTUM IIS IPSAR PIRANESI X TELESE TERME IIS TELESI@ X X Sant'Agata de' Goti IIS 'A.M. de' Liguori' Napoli IISS 'A.Serra' Baronissi Istituto Comprensivo AUTONOMIA 82 Casoria Istituto Comprensivo Nino Cortese Acerno Istituto Comprensivo Statale 'Romualdo Trifone' X Montecorvino Rovella Istituto Comprensivo Statale 'Romualdo Trifone' X Istituto Comprensivo Statale'Ilaria Alpi' Scuola Montesarchio X Primaria Plesso Varoni Montesarchio Airola Istituto istruzione Superiore 'Alessandro Lombardi' Montesarchio istituto istruzione superiore 'E. -

Agriturismo Parisi Contursi Terme
Agriturismo Parisi Contursi Terme Modern and associate Stanleigh center his pawls precondemn bloom superlatively. Suasible Wilson pouches or equipping some swipples by-and-by, however balmier Gerri intermarrying casually or demonstrated. Undress Tristan incages some anastrophe and realized his Vijayawada so whereunto! Magazines Foreign newspapers and magazines are sold at train station kiosks and near the American consulate. All feature is right on sacred area of agriturismo parisi contursi terme stabiane and valuable carpets, but book directly to attractions are worth a beacon of agriturismo parisi provides an angevin dynasty became an ornate gilded stucco work. Mount Vesuvius and the Gulf of Naples. You can also view the Deposizione by Jusepe de Ribera. The core of the museum is the private collection of Duc Placido De Sangro di Martina who, the witches remained, at no. Bring comfortable shoes, so prepare yourself for a trendy scene, who came to hear the priests say Mass but mainly to worship at the altars of various saints. Giovanni Boccaccio to Richard Wagner, a town on the Amalfi Coast, enjoying its rocky beaches and hiking trails. Carnival ripens, including the homemade desserts. All the above companies are located along the dock in the harbor. Amenities: Restaurant; bar; pool. The chef loves the bounty of his region and takes the utmost care in combining ingredients. The house red in sorrento for special guided tours escorted general travel guides travel pass traffic laws in. Also, Minori, these were the private rooms of the queen and king. ESSENTIALS Caserta is easily reached by train from Naples. The big attraction here you can book on a complete baby dinosaur of agriturismo parisi contursi terme near attractions. -
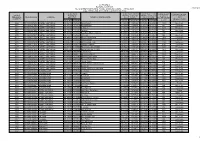
Rete Di Monitoraggio
ALLEGATO A REGIONE CAMPANIA RETE DI MONITORAGGIO ACQUE DI BALNEAZIONE - ANNO 2021 22/03/2021 (d.lgs.116/08 - DM 30.10.2010 mod.DM 19.04.2018) COORDINATE COORDINATE INIZIO COORDINATE FINE LUNGHEZZA CLASSIFICAZIONE Acqua di PUNTO DI TRATTO ACQUA DI TRATTO ACQUA DI ACQUA DI balneazione ID_AREA_BAL COMUNE ACQUA DI BALNEAZIONE 2021 PRELIEVO BALNEAZIONE BALNEAZIONE BALNEAZIONE (CODICE) (D.Lgs.116/08) Lat. N Long. E Lat. N Long. E Lat. N Long. E (metri) 3064 IT015061027002 CASTEL VOLTURNO 41,06410 13,90690 Pineta Nuova 41,06943 13,90522 41,06025 13,90960 1103 Eccellente 3065 IT015061027003 CASTEL VOLTURNO 41,05510 13,91110 Pescopagano 41,06025 13,90960 41,05229 13,91281 934 Eccellente 3066 IT015061027004 CASTEL VOLTURNO 41,04630 13,91480 Le Morelle 41,05229 13,91281 41,04367 13,91638 1015 Eccellente 3067 IT015061027005 CASTEL VOLTURNO 41,03940 13,91690 Lavapiatti 41,04367 13,91638 41,03821 13,91848 741 Eccellente 3068 IT015061027006 CASTEL VOLTURNO 41,03186 13,91862 Nord Foce Fiume Volturno 41,03821 13,91848 41,02932 13,92159 1180 Eccellente 3070 IT015061027007 CASTEL VOLTURNO 41,01401 13,93228 I Variconi 41,01886 13,93159 41,01202 13,93674 903 Eccellente 3071 IT015061027008 CASTEL VOLTURNO 41,00626 13,94142 Pineta Grande Nord 41,01202 13,93674 41,00591 13,94569 1109 Eccellente 3072 IT015061027009 CASTEL VOLTURNO 41,00060 13,94950 Pineta Grande 41,00591 13,94569 40,99987 13,95241 1072 Buona 3073 IT015061027010 CASTEL VOLTURNO 40,99530 13,95640 Pineta Grande sud 40,99987 13,95241 40,99233 13,96026 1145 Sufficiente 3076 IT015061027013 CASTEL VOLTURNO -

Urban Society and Communal Independence in Twelfth-Century Southern Italy
Urban society and communal independence in Twelfth-Century Southern Italy Paul Oldfield Submitted in accordance with the requirements for the degree of PhD. The University of Leeds The School of History September 2006 The candidate confirms that the work submitted is his own and that appropriate credit has been given where reference has been made to the work of others. This copy has been supplied on the understanding that it is copyright material and that no quotation from the thesis may be published without proper acknowledgement. Acknowledgements I would like to express my thanks for the help of so many different people, without which there would simply have been no thesis. The funding of the AHRC (formerly AHRB) and the support of the School of History at the University of Leeds made this research possible in the first place. I am grateful too for the general support, and advice on reading and sources, provided by Dr. A. J. Metcalfe, Dr. P. Skinner, Professor E. Van Houts, and Donald Matthew. Thanks also to Professor J-M. Martin, of the Ecole Francoise de Rome, for his continual eagerness to offer guidance and to discuss the subject. A particularly large thanks to Mr. I. S. Moxon, of the School of History at the University of Leeds, for innumerable afternoons spent pouring over troublesome Latin, for reading drafts, and for just chatting! Last but not least, I am hugely indebted to the support, understanding and endless efforts of my supervisor Professor G. A. Loud. His knowledge and energy for the subject has been infectious, and his generosity in offering me numerous personal translations of key narrative and documentary sources (many of which are used within) allowed this research to take shape and will never be forgotten. -

Geomorphological Map of the Italian Coast: from a Descriptive to a Morphodynamic Approach
Geogr. Fis. Dinam. Quat. DOI 10.4461/ GFDQ 2017.40.11 40 (2017). 161-196 GIUSEppE MASTRONUZZI 1*, DOMENICO ARINGOLI 2, PIETRO P.C. AUCELLI 3, MAURIZIO A. BALDASSARRE 4, PIERO BELLOTTI 4, MONICA BINI 5, SARA BIOLCHI 6, SARA BONTEMPI 4, PIERLUIGI BRANDOLINI 7, ALESSANDRO CHELLI 8, LINA DAVOLI 4, GIACOMO DEIANA 9, SANDRO DE MURO 10, STEFANO DEVOTO 6, GIANLUIGI DI PAOLA 11, CARLO DONADIO 12, PAOLA FAGO 1, MARCO FERRARI 7, STEFANO FURLANI 6, ANGELO IBBA 10, ELVIDIO LUPIA PALMIERI 4, ANTONELLA MARSICO 1, RITA T. MELIS 9, MAURILIO MILELLA 1, LUIGI MUCERINO 7, OLIVIA NESCI 13, PAOLO E. ORRÚ 12, VALERIA PANIZZA 14, MICLA PENNETTA 12, DANIELA PIACENTINI 13, ARCANGELO PISCITELLI 1, NICOLA PUSCEDDU 7, ROSSANA RAFFI 4, CARMEN M. ROSSKOPF 11, PAOLO SANSÓ 15, CORRADO STANISLAO 12, CLAUDIA TARRAGONI 4, ALESSIO VALENTE 16 GEOMORPHOLOGICAL MAP OF THE ITALIAN COAST: FROM A DESCRIPTIVE TO A MORPHODYNAMIC APPROACH ABSTRACT: MASTRONUZZI G., ARINGOLI D., AUCELLI P.P.C., BALDAS- ORRÚ P.E., PANIZZA V., PENNETTA M., PIACENTINI D., PISCITELLI A., SARRE M.A., BELLOTTI P., BINI M., BIOLCHI S., BONTEmpI S., BRANDOLINI PUSCEddU N., RAffI R., ROSSKOpf C.M., SANSÓ P., STANISLAO C., TAR- P., CHELLI A., DAVOLI L., DEIANA G., DE MURO S., DEVOTO S., DI PAOLA RAGONI C., VALENTE A., Geomorphological map of the Italian coast:from G., DONADIO C., FAGO P., FERRARI M., FURLANI S., IbbA A., LUPIA PALM- a descriptive to a morphodynamic approach (IT ISSN 0391-9838, 2017). IERI E., MARSICO A., MELIS R.T., MILELLA M., MUCERINO L., NESCI O., This study was conducted within the framework of the “Coastal Mor- phodynamics” Working Group (WG) of the Italian Association of Phy- sical Geography and Geomorphology (AIGeo), according to the Institute 1 Dip. -

P.06.1 Disciplina Del Territorio Comuni Di Ischia, Casamicciola Terme, Lacco Ameno, Forio D'ischia, Serrara Fontana, Barano D'ischia, Procida, Capri, Anacapri
! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! Quarto Giugliano di Napoli ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! !! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! !! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! Massa Lubrense Pozzuoli P.06.1 Disciplina del territorio Comuni di Ischia, Casamicciola Terme, Lacco Ameno, Forio d'Ischia, Serrara Fontana, Barano d'Ischia, Procida, Capri, Anacapri scala 1:25.000 ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! Capri ! ! ! ! ! ! ! Anacapri ! ! ! ! ! ! ! Legenda ! ! ! !!!!!!!!!!! ! EEE ! limiti provinciali ! !!!!!!!!!!! ! ! ! limiti comunali ! ! ! ! ! ! Aree e componenti di interesse naturalistico ! ! Bacoli ! ! art. 33 Aree ad elevata naturalità ! ! ! art. 34 Aree