Variable Susceptibility to an Emerging Infectious Disease, Chytridiomycosis, in Anurans
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Herpetological Journal FULL PAPER
Volume 29 (April 2019), 71-81 Herpetological Journal FULL PAPER https://doi.org/10.33256/hj29.2.7181 Published by the British Predicting Ambystoma ordinarium distribution under differentHerpetological Society climate scenarios in central Mexico Rafael Hernández-Guzmán1, Luis H. Escalera-Vázquez2 & Ireri Suazo-Ortuño3 1CONACYT – Instituto de Investigaciones sobre los Recursos Naturales, Universidad Michoacana de San Nicolás de Hidalgo. Morelia, Michoacán, México 2Laboratorio de Biología Acuática, Facultad de Biología, Universidad Michoacana de San Nicolás de Hidalgo, edificio R, planta baja, Ciudad Universitaria. Morelia, Michoacán, México 3Instituto de Investigaciones sobre los Recursos Naturales, Universidad Michoacana de San Nicolás de Hidalgo. Morelia, Michoacán, México Global climate change represents one of the most important threats to wildlife populations. Amphibians, specifically salamanders, are particularly susceptible to the effects of a changing climate due to their restrictive physiological requirements and low vagility; however, little is known about which amphibian species are more vulnerable to climate change. Therefore, we aimed to forecast changes in the distribution of the mountain stream salamander, Ambystoma ordinarium, using different climate scenarios. Approximately 70 representative presence records were selected to model the current potential distribution and two scenarios based on 2070 climate projections (RCP 2.6 and RCP 8.5) using the MaxEnt algorithm and three global climate models (BCC-CSM1-1, CCSM4 and HadGEM2-ES). A total of three scenarios were simulated using the 10-percentile training presence as the threshold rule. For all scenarios, the average of the area under the receiver operating characteristic curve for the replicated runs was greater than 0.95 ± 0.005, representing good performance for the current and projected geographical distributions of A. -

Darwin Initiative for the Survival of Species Final Report 1. Darwin
Final Report on The Atelopus Initiative: Conserving Endangered Tropical Andean Amphibians Darwin Initiative for the Survival of Species Final Report 1. Darwin Project Information Project Reference No. 13-017 (formerly 268) Project title The Atelopus Initiative: Conserving Endangered Tropical Andean Amphibians Country Venezuela, Colombia, Ecuador, Perú and Bolivia UK Contractor Conservation International-UK Partner Organisation (s) THE ATELOPUS INITIATIVE is a multi-national partnership of herpetologists from the following institutions: Conservation International, The Natural History Museum, IUCN, NatureServe, and many US, European and Andean institutions. Darwin Grant Value £ 186,695 Start/End date April 1, 2004 – March 31, 2007 Project website www.andescbc.org/atelopus Author(s), date Ariadne Angulo, José Vicente Rodríguez-Mahecha, Patricio Jarrin, Robert Bensted-Smith 2. Project Background/Rationale • Describe the location and circumstances of the project The Atelopus Initiative is a regional project funded by a generous grant by the Darwin Initiative (DI), encompassing the five tropical Andean nations of Venezuela, Colombia, Ecuador, Peru and Bolivia. Following the results of the Global Amphibian Assessment (GAA) and the National Assessment processes developed in Venezuela, Colombia and Peru, which found that almost one third of all known amphibian species are currently considered to be threatened, the project team proposed the Atelopus Initiative as a response to address the amphibian population declines and extinction crisis in the tropical Andes. The crisis is of special concern in the region, given that it is considered to be a biodiversity hotspot and epicentre of amphibian richness especially in he upper Amazon basin. 1 Conservation International Final Report on The Atelopus Initiative: Conserving Endangered Tropical Andean Amphibians • What was the problem that the project aimed to address? The project aimed to address the amphibian population declines and extinction crisis in the tropical Andes Countries. -

A Collection of Amphibians from Río San Juan, Southeastern Nicaragua
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/264789493 A collection of amphibians from Río San Juan, southeastern Nicaragua Article in Herpetology Notes · January 2009 CITATIONS READS 12 188 4 authors, including: Javier Sunyer Matthias Dehling University of Canterbury 89 PUBLICATIONS 209 CITATIONS 54 PUBLICATIONS 967 CITATIONS SEE PROFILE SEE PROFILE Gunther Köhler Senckenberg Research Institute 222 PUBLICATIONS 1,617 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Zoological Research in Strict Forest Reserves in Hesse, Germany View project Diploma Thesis View project All content following this page was uploaded by Javier Sunyer on 16 August 2018. The user has requested enhancement of the downloaded file. Herpetology Notes, volume 2: 189-202 (2009) (published online on 29 October 2009) A collection of amphibians from Río San Juan, southeastern Nicaragua Javier Sunyer1,2,3*, Guillermo Páiz4, David Matthias Dehling1, Gunther Köhler1 Abstract. We report upon the amphibians collected during seven expeditions carried out between the years 2000–2006 to thirteen localities in both Refugio de Vida Silvestre Río San Juan and Reserva Biológica Indio-Maíz, southeastern Nicaragua. We include morphometric data of around one-half of the adult specimens in the collection, and provide a brief general overview and discuss zoogeographic and conservation considerations of the amphibians known to occur in the Río San Juan area. Keywords. Amphibia, conservation, ecology, morphometry, zoogeography. Introduction potential of holding America’s first interoceanic channel and also because it was part of the sea route to travel The San Juan River is an approximately 200 km slow- from eastern to western United States. -

9-Altıncı Yok Oluş, Yazar: Elizabeth Kolbert
ELIZABETH KOLBERT okuyanj^fus PULITZER ODULU SAHİBİ NEW YORK TIMES BOOK REVlEVI/’k GÖRE YILIN EN İYİ 10 KİTABINDAN BİRİ NEW YORK TIMESBESTSELLER NATIONAL BOOK CRITICS CIRCLE ODULU FİNALİSTİ Son yarım milyar yılda tam beş kitlesel yok oluş yaşandı; dünyada yaşam çeşitli liği aniden ve dramatik ölçüde azaldı. Peki gözlerimizin önünde yeni bir kitlesel yok oluş yaşanıyor olabilir mi? "Altıncı Yok Oluş harika bir kitap... Büyük, ani değişikliklerin yaşanabileceğini, bunun olasılık dışı olmadığını açıkça ortaya koyuyor. Bunlar daha önce yaşandı, yeniden yaşanabilir." —ABD Başkam Barack Obama Dünyanın dört yanında bilim insanları, dinozorların yok olmasına neden olan asteroit çarpmasından sonra en yıkıcı yok oluş süreci olduğunu öngördükleri altıncı yok oluşu gözlemliyor. Bu kez, felaketin nedeni biziz. "Doğa bilimciler dünya tarihinde beş yok oluş gerçekleştiğini söyler; Kolbert, insan faaliyetlerinin, gezegeni altıncı yok oluşa götürdüğüne dair ikna edici bir tez ortaya koyuyor." —Bili Gates Hem samimi, hem eğlenceli, hem de bilgi dolu bu kitapta, Neıo Yorker yazarı Eliza- beth Kolbert, insanın, gezegenimizdeki hayatı, diğer hiçbir türün yapmadığı şe kilde değiştirmesinin nedenini ve nasılını anlatıyor. Çok sayıda disiplinde yapıl mış araştırmaları, yok olmuş türlerin tanımlarını ve kavram olarak yok oluşun geçmişini bir araya getiren Kolbert, gözlerimizin önünde yok olmakta olan türlere dair etkileyici ve kapsamlı bir hikaye sunuyor. Kolbert, altıncı yok oluşun insanoğ lunun en kalıcı mirası olmaya aday olduğunu gösteriyor ve bizleri -

Catalogue of the Amphibians of Venezuela: Illustrated and Annotated Species List, Distribution, and Conservation 1,2César L
Mannophryne vulcano, Male carrying tadpoles. El Ávila (Parque Nacional Guairarepano), Distrito Federal. Photo: Jose Vieira. We want to dedicate this work to some outstanding individuals who encouraged us, directly or indirectly, and are no longer with us. They were colleagues and close friends, and their friendship will remain for years to come. César Molina Rodríguez (1960–2015) Erik Arrieta Márquez (1978–2008) Jose Ayarzagüena Sanz (1952–2011) Saúl Gutiérrez Eljuri (1960–2012) Juan Rivero (1923–2014) Luis Scott (1948–2011) Marco Natera Mumaw (1972–2010) Official journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 13(1) [Special Section]: 1–198 (e180). Catalogue of the amphibians of Venezuela: Illustrated and annotated species list, distribution, and conservation 1,2César L. Barrio-Amorós, 3,4Fernando J. M. Rojas-Runjaic, and 5J. Celsa Señaris 1Fundación AndígenA, Apartado Postal 210, Mérida, VENEZUELA 2Current address: Doc Frog Expeditions, Uvita de Osa, COSTA RICA 3Fundación La Salle de Ciencias Naturales, Museo de Historia Natural La Salle, Apartado Postal 1930, Caracas 1010-A, VENEZUELA 4Current address: Pontifícia Universidade Católica do Río Grande do Sul (PUCRS), Laboratório de Sistemática de Vertebrados, Av. Ipiranga 6681, Porto Alegre, RS 90619–900, BRAZIL 5Instituto Venezolano de Investigaciones Científicas, Altos de Pipe, apartado 20632, Caracas 1020, VENEZUELA Abstract.—Presented is an annotated checklist of the amphibians of Venezuela, current as of December 2018. The last comprehensive list (Barrio-Amorós 2009c) included a total of 333 species, while the current catalogue lists 387 species (370 anurans, 10 caecilians, and seven salamanders), including 28 species not yet described or properly identified. Fifty species and four genera are added to the previous list, 25 species are deleted, and 47 experienced nomenclatural changes. -

Chec List a Checklist of the Amphibians and Reptiles of San
Check List 10(4): 870–877, 2014 © 2014 Check List and Authors Chec List ISSN 1809-127X (available at www.checklist.org.br) Journal of species lists and distribution PECIES S OF A checklist * of the amphibians and reptiles of San Isidro de ISTS L Dota, Reserva Forestal Los Santos, Costa Rica Erick Arias and Federico Bolaños [email protected] Universidad de Costa Rica, Escuela de Biología, Museo de Zoología. San Pedro, 11501-2060, San José, Costa Rica. * Corresponding author. E-mail: Abstract: We present an inventory of amphibians and reptiles of San Isidro de Dota, northwest of the Cordillera de Talamanca in the Central Pacific of Costa Rica.Leptodactylus The study was insularum conduced from January to August 2012 in premontane wet Coloptychonforest from 689 rhombifer m to 800 m elevation. We found a total of 56 species, including 30 species of amphibians and 26 of reptiles. It results striking the presence of the frog , uncommon above 400 m elevation, and the lizard , a very uncommon species. DOI: 10.15560/10.4.870 Introduction datum, from 689 m to 800 N, 83°58′32.41″ W, WGS84et al. Lower Central America represents one of the regions m elevation). The region is dominated by premontane with the highest numberet al of amphibianset describedal. in the wet forest (Bolaños 1999) with several sites used Neotropics in relation to the area it represent (Savage for agriculture and pastures. The region presents the 2002; Boza-Oviedo . 2012; Hertz 2012). Much climate of the pacific slope of the Cordillera de Talamanca, of this richness of species iset associated al. -

Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca
Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca John F. Lamoreux, Meghan W. McKnight, and Rodolfo Cabrera Hernandez Occasional Paper of the IUCN Species Survival Commission No. 53 Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca John F. Lamoreux, Meghan W. McKnight, and Rodolfo Cabrera Hernandez Occasional Paper of the IUCN Species Survival Commission No. 53 The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily reflect those of IUCN or other participating organizations. Published by: IUCN, Gland, Switzerland Copyright: © 2015 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Citation: Lamoreux, J. F., McKnight, M. W., and R. Cabrera Hernandez (2015). Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca. Gland, Switzerland: IUCN. xxiv + 320pp. ISBN: 978-2-8317-1717-3 DOI: 10.2305/IUCN.CH.2015.SSC-OP.53.en Cover photographs: Totontepec landscape; new Plectrohyla species, Ixalotriton niger, Concepción Pápalo, Thorius minutissimus, Craugastor pozo (panels, left to right) Back cover photograph: Collecting in Chamula, Chiapas Photo credits: The cover photographs were taken by the authors under grant agreements with the two main project funders: NGS and CEPF. -
For Review Only
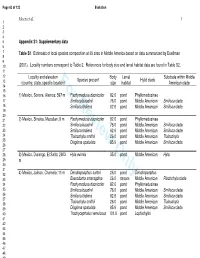
Page 63 of 123 Evolution Moen et al. 1 1 2 3 4 5 Appendix S1: Supplementary data 6 7 Table S1 . Estimates of local species composition at 39 sites in Middle America based on data summarized by Duellman 8 9 10 (2001). Locality numbers correspond to Table 2. References for body size and larval habitat data are found in Table S2. 11 12 Locality and elevation Body Larval Subclade within Middle Species present Hylid clade 13 (country, state, specific location)For Reviewsize Only habitat American clade 14 15 16 1) Mexico, Sonora, Alamos; 597 m Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 17 Smilisca baudinii 76.0 pond Middle American Smilisca clade 18 Smilisca fodiens 62.6 pond Middle American Smilisca clade 19 20 21 2) Mexico, Sinaloa, Mazatlan; 9 m Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 22 Smilisca baudinii 76.0 pond Middle American Smilisca clade 23 Smilisca fodiens 62.6 pond Middle American Smilisca clade 24 Tlalocohyla smithii 26.0 pond Middle American Tlalocohyla 25 Diaglena spatulata 85.9 pond Middle American Smilisca clade 26 27 28 3) Mexico, Durango, El Salto; 2603 Hyla eximia 35.0 pond Middle American Hyla 29 m 30 31 32 4) Mexico, Jalisco, Chamela; 11 m Dendropsophus sartori 26.0 pond Dendropsophus 33 Exerodonta smaragdina 26.0 stream Middle American Plectrohyla clade 34 Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 35 Smilisca baudinii 76.0 pond Middle American Smilisca clade 36 Smilisca fodiens 62.6 pond Middle American Smilisca clade 37 38 Tlalocohyla smithii 26.0 pond Middle American Tlalocohyla 39 Diaglena spatulata 85.9 pond Middle American Smilisca clade 40 Trachycephalus venulosus 101.0 pond Lophiohylini 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 Evolution Page 64 of 123 Moen et al. -

Dedicated to the Conservation and Biological Research of Costa Rican Amphibians”
“Dedicated to the Conservation and Biological Research of Costa Rican Amphibians” A male Crowned Tree Frog (Anotheca spinosa) peering out from a tree hole. 2 Text by: Brian Kubicki Photography by: Brian Kubicki Version: 3.1 (October 12th, 2009) Mailing Address: Apdo. 81-7200, Siquirres, Provincia de Limón, Costa Rica Telephone: (506)-8889-0655, (506)-8841-5327 Web: www.cramphibian.com Email: [email protected] Cover Photo: Mountain Glass Frog (Sachatamia ilex), Quebrada Monge, C.R.A.R.C. Reserve. 3 Costa Rica is internationally recognized as one of the most biologically diverse countries on the planet in total species numbers for many taxonomic groups of flora and fauna, one of those being amphibians. Costa Rica has 190 species of amphibians known from within its tiny 51,032 square kilometers territory. With 3.72 amphibian species per 1,000 sq. km. of national territory, Costa Rica is one of the richest countries in the world regarding amphibian diversity density. Amphibians are under constant threat by contamination, deforestation, climatic change, and disease. The majority of Costa Rica’s amphibians are surrounded by mystery in regards to their basic biology and roles in the ecology. Through intense research in the natural environment and in captivity many important aspects of their biology and conservation can become better known. The Costa Rican Amphibian Research Center (C.R.A.R.C.) was established in 2002, and is a privately owned and operated conservational and biological research center dedicated to studying, understanding, and conserving one of the most ecologically important animal groups of Neotropical humid forest ecosystems, that of the amphibians. -

NORTHWEST NAZARENE UNIVERSITY Assisting Frog
NORTHWEST NAZARENE UNIVERSITY Assisting Frog Identification in Costa Rica Using a Mobile App THESIS Submitted to the Department of Mathematics and Computer Science in partial fulfillment of the requirements for the degree of BACHELOR OF ARTS Justin Tyler Laplante 2021 THESIS Submitted to the Department of Mathematics and Computer Science in partial fulfillment of the requirements for the degree of BACHELOR OF ARTS Justin Tyler Laplante 2021 Assisting Frog Identification in Costa Rica Using a Mobile App Author: ____________________________________________________________ Justin Tyler Laplante Approved: ____________________________________________________________ Dale Hamilton, Ph.D., Professor, Department of Mathematics and Computer Science, Faculty Advisor Approved: ____________________________________________________________ John Cossel Jr., Ph.D., Professor, Chair, Department of Biology Second Reader Approved: ____________________________________________________________ Barry L. Myers, Ph.D., Chair, Department of Mathematics & Computer Science ABSTRACT Assisting Frog Identification in Costa Rica Using a Mobile App. LAPLANTE, JUSTIN (Department of Mathematics and Computer Science). Quickly identifying a single frog species from over a hundred other possible species can be a challenge for research while in the Costa Rican jungle. Though researchers can use field guides to assist, these still mean you may have look through all currently identified frog species to find the frog being viewed. This project was created to help researchers narrow the list of possible frog species quickly based on Geolocation. Using Xamarin.Forms, an app was developed that worked offline, used an ArcGIS API and was cross platform. However, to ensure performs and accuracy certain design choices were made for designing the ArcGIS map that was used within the app. The used geospatial data for the frog species and generalized it into a hexagonal pattern. -

The Most Frog-Diverse Place in Middle America, with Notes on The
Offcial journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 13(2) [Special Section]: 304–322 (e215). The most frog-diverse place in Middle America, with notes on the conservation status of eight threatened species of amphibians 1,2,*José Andrés Salazar-Zúñiga, 1,2,3Wagner Chaves-Acuña, 2Gerardo Chaves, 1Alejandro Acuña, 1,2Juan Ignacio Abarca-Odio, 1,4Javier Lobon-Rovira, 1,2Edwin Gómez-Méndez, 1,2Ana Cecilia Gutiérrez-Vannucchi, and 2Federico Bolaños 1Veragua Foundation for Rainforest Research, Limón, COSTA RICA 2Escuela de Biología, Universidad de Costa Rica, San Pedro, 11501-2060 San José, COSTA RICA 3División Herpetología, Museo Argentino de Ciencias Naturales ‘‘Bernardino Rivadavia’’-CONICET, C1405DJR, Buenos Aires, ARGENTINA 4CIBIO Research Centre in Biodiversity and Genetic Resources, InBIO, Universidade do Porto, Campus Agrário de Vairão, Rua Padre Armando Quintas 7, 4485-661 Vairão, Vila do Conde, PORTUGAL Abstract.—Regarding amphibians, Costa Rica exhibits the greatest species richness per unit area in Middle America, with a total of 215 species reported to date. However, this number is likely an underestimate due to the presence of many unexplored areas that are diffcult to access. Between 2012 and 2017, a monitoring survey of amphibians was conducted in the Central Caribbean of Costa Rica, on the northern edge of the Matama mountains in the Talamanca mountain range, to study the distribution patterns and natural history of species across this region, particularly those considered as endangered by the International Union for Conservation of Nature. The results show the highest amphibian species richness among Middle America lowland evergreen forests, with a notable anuran representation of 64 species. -

Assessing Population Health of the Toluca Axolotl Ambystoma Rivulare (Taylor, 1940) from México, Using Leukocyte Profiles
Herpetological Conservation and Biology 10(2):592–601. Submitted: 29 May 2014; Accepted: 6 March 2015; Published: 31 August 2015. ASSESSING POPULATION HEALTH OF THE TOLUCA AXOLOTL AMBYSTOMA RIVULARE (TAYLOR, 1940) FROM MÉXICO, USING LEUKOCYTE PROFILES CARLOS BARRIGA-VALLEJO1,2, OSWALDO HERNÁNDEZ-GALLEGOS2, IONE HUNT VON HERBING3, ANA ESTHELA LÓPEZ-MORENO2, MARÍA DE LOURDES RUIZ-GÓMEZ2, GISELA GRANADOS-GONZALEZ2, MÓNICA VANESA GARDUÑO-PAZ2, JOSÉ FERNANDO MÉNDEZ- SÁNCHEZ2, JAVIER BANDA-LEAL4, AND ANDREW K. DAVIS5,6 1Laboratorio de Ecofisiología, Facultad de Ciencias Biológicas, Universidad Autónoma de Nuevo León, San Nicolás de los Garza, Apartado Postal - 513, C.P. 66450, Nuevo León, México 2Facultad de Ciencias, Universidad Autónoma del Estado de México, Instituto Literario 100, Toluca Centro, C.P. 50000, México 3Department of Biological Sciences, University of North Texas, Denton, Texas 76201 4Universidad Autónoma de Nuevo León, Facultad de Ciencias Biológicas, Laboratorio de Herpetología, Apartado Postal # 513, San Nicolás de los Garza, Nuevo León, C.P. C.P. 66450, México 5Odum School of Ecology, University of Georgia, Athens, Georgia, USA 30602 6Corresponding author, e-mail: [email protected] Abstract.—World-wide declines of amphibians have heightened the need for information relating to their health status and immune function under natural conditions. Evaluation of differential white blood cell (leukocyte) counts from thin blood smears is one way to gain this information, and this approach is increasingly being used by herpetologists to gauge the integrity of amphibian populations. This approach is especially useful in natural settings because amphibian leucocyte profiles can vary depending on biological and physiological processes, including those caused by environmental factors.