The Relation Between Cesarean Birth and Child Cognitive Development Received: 10 March 2017 Cain Polidano1, Anna Zhu1 & Joel C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Brass Bands of the World a Historical Directory
Brass Bands of the World a historical directory Kurow Haka Brass Band, New Zealand, 1901 Gavin Holman January 2019 Introduction Contents Introduction ........................................................................................................................ 6 Angola................................................................................................................................ 12 Australia – Australian Capital Territory ......................................................................... 13 Australia – New South Wales .......................................................................................... 14 Australia – Northern Territory ....................................................................................... 42 Australia – Queensland ................................................................................................... 43 Australia – South Australia ............................................................................................. 58 Australia – Tasmania ....................................................................................................... 68 Australia – Victoria .......................................................................................................... 73 Australia – Western Australia ....................................................................................... 101 Australia – other ............................................................................................................. 105 Austria ............................................................................................................................ -

Scientists' Houses in Canberra 1950–1970
EXPERIMENTS IN MODERN LIVING SCIENTISTS’ HOUSES IN CANBERRA 1950–1970 EXPERIMENTS IN MODERN LIVING SCIENTISTS’ HOUSES IN CANBERRA 1950–1970 MILTON CAMERON Published by ANU E Press The Australian National University Canberra ACT 0200, Australia Email: [email protected] This title is also available online at http://epress.anu.edu.au National Library of Australia Cataloguing-in-Publication entry Author: Cameron, Milton. Title: Experiments in modern living : scientists’ houses in Canberra, 1950 - 1970 / Milton Cameron. ISBN: 9781921862694 (pbk.) 9781921862700 (ebook) Notes: Includes bibliographical references and index. Subjects: Scientists--Homes and haunts--Australian Capital Territority--Canberra. Architecture, Modern Architecture--Australian Capital Territority--Canberra. Canberra (A.C.T.)--Buildings, structures, etc Dewey Number: 720.99471 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying or otherwise, without the prior permission of the publisher. Cover design by Sarah Evans. Front cover photograph of Fenner House by Ben Wrigley, 2012. Printed by Griffin Press This edition © 2012 ANU E Press; revised August 2012 Contents Acknowledgments . vii Illustrations . xi Abbreviations . xv Introduction: Domestic Voyeurism . 1 1. Age of the Masters: Establishing a scientific and intellectual community in Canberra, 1946–1968 . 7 2 . Paradigm Shift: Boyd and the Fenner House . 43 3 . Promoting the New Paradigm: Seidler and the Zwar House . 77 4 . Form Follows Formula: Grounds, Boyd and the Philip House . 101 5 . Where Science Meets Art: Bischoff and the Gascoigne House . 131 6 . The Origins of Form: Grounds, Bischoff and the Frankel House . 161 Afterword: Before and After Science . -

Grounds, Boyd and the Philip House
4. Form Follows Formula: Grounds, Boyd and the Philip House Figure 4.1 Philip House, view from north-east Photograph: Ben Wrigley, 2011 John Philip was brought to Canberra as part of Frankel’s ambitious postwar recruitment program, and was appointed head of a new agricultural physics group at the CSIRO. Regarded as Australia’s leading environmental physicist, he was elected a Fellow of the Australian Academy of Science in 1967. His wife, Frances (‘Fay’), was an accomplished artist who was related to the Boyds via the Mills and à Becketts, and had attended the Murrumbeena State School in Victoria with Mary and Arthur Boyd. Many of Frances’s portraits of Australia’s leading scientists and academics—including Sir Mark Oliphant, Doug Waterhouse, John Jaeger, William Rogers, Patrick Moran and Manning Clark—are held in the collections of the Australian Academy of Science and The Australian National University. The Philip House, at 42 Vasey Crescent, Campbell, is one of three adjacent houses by Grounds, Romberg and Boyd that are known collectively as the Vasey Crescent Group. The other two houses in the group are the Blakers House and the Griffing House. Grounds and Boyd were both involved with these houses. All three were designed by Grounds, who arranged initial briefings, recorded 101 Experiments in Modern Living the clients’ requirements and prepared sketches from late 1959 through to early 1960. Boyd met with the clients in January 1960, and took control of the houses from May of that year as Grounds prepared for a three-month overseas trip.1 The Philip House is important for two reasons. -

Lands of Tasmania" an E1tor Was Made in Each of These Averages, B
(No. 28.) 18 6 4. TASMANIA. L E G I S L A T I V E C O U N C 1 L. L A N D S OF T A S M A N I A. Laid on the Table by Mr. Whyte, and ordered by the Council to be printed, July 1, 1864. .. OF TAS1\1ANIA; COMPILED FROM THE OF~CIAL RECORDS OF THE SURVEY DEPARTMENT, BY ORDER OF THE HONORABLE THE COLONIAL TREASURER Made up to the 31st December, 1862. «ar;mani,t: JAMES BARNARD, GOVERNMENT PRINTER, HOBART TOWN. \ 18 6 4. T A B LE OF C O N T E N T S. PAGE PREFACE •••••.••••••••••••••••••• 3 Area of Tasmania, with alienated and unalienated Lands ...........••... , • . 17 Population of Tasmania •. , ..... , . • . • • . • • . • . • . • . ib. Ditto of Towns .................•••.........•.......... _. 18 · Country Lands granted and sold since 1804 ..•• , •• , ..•....•....... , . • • • . 19 Town Lands sold ..••••......•.......••••...••• , . • . 20 'fown Lands sold for Cash under " The Waste Lands Act" . • • • • • • . 21 Deposits forfeited on ditto. • • • • • • . • . ... , . • • . • . • . 40 Town Lands sold on Credit .......... , ......••.. , , ......... , ..•.... , . , . 42 Agricultuml Lands sold for Cash, under 18th Sect. of '' The Waste Lands Act". 4'5 Ditto on Credit, ditto ...• .', . • . • . • • • • . • . • 46 Ditto for Cash, under 19th Sect. of" The Waste Lands Act" . 49 Ditto on Credit, ditto ....•••••.•....... , , ....... , ....• •... , . • • • • • . 51 Ditto for Cash at Public Auction .••••.............•••.••. , , • . 62 Deposits forfeited on ditto ...... , ........• , .......•.. , . • . 64 Agricultural Lands sold on Credit at Public Auction , •.•••••..•••••.• , . 65 Pastoral Lands sold for CashJ under 18th Sect. of" The ·waste Lands Act" .. , . 71 Ditto on Credit, ditto .•••...•....••..••..•..••............• , • . • • . ib. Ditto for Cash at Public Auction ....•.•.•.•...... , . • • . • . • • . • . 73 Deposits forfeited on ditto •.••••............•., • , • • . • • • . • • • . 74 Pastoral Lands sold on Credit at Public Auction...... -
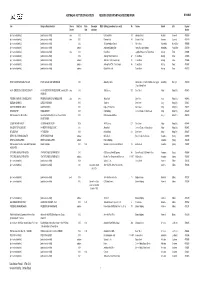
AIA REGISTER Jan 2015
AUSTRALIAN INSTITUTE OF ARCHITECTS REGISTER OF SIGNIFICANT ARCHITECTURE IN NSW BY SUBURB Firm Design or Project Architect Circa or Start Date Finish Date major DEM Building [demolished items noted] No Address Suburb LGA Register Decade Date alterations Number [architect not identified] [architect not identified] circa 1910 Caledonia Hotel 110 Aberdare Street Aberdare Cessnock 4702398 [architect not identified] [architect not identified] circa 1905 Denman Hotel 143 Cessnock Road Abermain Cessnock 4702399 [architect not identified] [architect not identified] 1906 St Johns Anglican Church 13 Stoke Street Adaminaby Snowy River 4700508 [architect not identified] [architect not identified] undated Adaminaby Bowling Club Snowy Mountains Highway Adaminaby Snowy River 4700509 [architect not identified] [architect not identified] circa 1920 Royal Hotel Camplbell Street corner Tumut Street Adelong Tumut 4701604 [architect not identified] [architect not identified] 1936 Adelong Hotel (Town Group) 67 Tumut Street Adelong Tumut 4701605 [architect not identified] [architect not identified] undated Adelonia Theatre (Town Group) 84 Tumut Street Adelong Tumut 4701606 [architect not identified] [architect not identified] undated Adelong Post Office (Town Group) 80 Tumut Street Adelong Tumut 4701607 [architect not identified] [architect not identified] undated Golden Reef Motel Tumut Street Adelong Tumut 4701725 PHILIP COX RICHARDSON & TAYLOR PHILIP COX and DON HARRINGTON 1972 Akuna Bay Marina Liberator General San Martin Drive, Ku-ring-gai Akuna Bay Warringah -
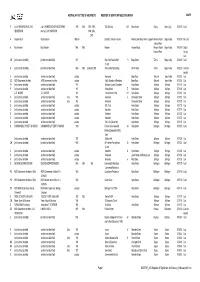
MASTER AIA Register of Significant Architecture February2021.Xls AUSTRALIAN INSTITUTE of ARCHITECTS REGISTER of SIGNIFICANT BUILDINGS in NSW MASTER
AUSTRALIAN INSTITUTE OF ARCHITECTS REGISTER OF SIGNIFICANT BUILDINGS IN NSW MASTER O A & K HENDERSON / LOUIS A & K HENDERSON OF MELBOURNE, 1935 1940 1991, 1993, T&G Building 555 Dean Street Albury Albury City 4703473Card HENDERSON rear by LOUIS HARRISON 1994, 2006, 2008 H Graeme Gunn Graeme Gunn 1968-69 Baronda (Yencken House) Nelson Lake Road, Nelson Lagoon Mimosa Rocks Bega Valley 4703519 No Card National Park H Roy Grounds Roy Grounds 1964 1980 Penders Haighes Road Mimosa Rocks Bega Valley 4703518 Digital National Park Listing Card CH [architect not identified] [architect not identified] 1937 Star of the Sea Catholic 19 Bega Street Tathra Bega Valley 4702325 Card Church G [architect not identified] [architect not identified] 1860 1862 Extended 2004 Tathra Wharf & Building Wharf Road Tathra Bega Valley 4702326 Card not located H [architect not identified] [architect not identified] undated Residence Bega Road Wolumla Bega Valley 4702327 Card SC NSW Government Architect NSW Government Architect undated Public School and Residence Bega Road Wolumla Bega Valley 4702328 Card TH [architect not identified] [architect not identified] 1911 Bellingen Council Chambers Hyde Street Bellingen Bellingen 4701129 Card P [architect not identified] [architect not identified] 1910 Federal Hotel 77 Hyde Street Bellingen Bellingen 4701131 Card I G. E. MOORE G. E. MOORE 1912 Former Masonic Hall 121 Hyde Street Bellingen Bellingen 4701268 Card H [architect not identified] [architect not identified] circa 1905 Residence 4 Coronation Street Bellingen Bellingen -

Mimosa Rocks National Park Plan of Management
Mimosa Rocks National Park Plan of Management MIMOSA ROCKS NATIONAL PARK PLAN OF MANAGEMENT NSW National Parks and Wildlife Service Part of the Department of Environment, Climate Change and Water February 2011 This plan of management was adopted by the Minister for Climate Change and the Environment on 2nd February 2011. Acknowledgments This plan of management is based on a draft plan prepared by staff of the Far South Coast Region of the NSW National Parks and Wildlife Service (NPWS), part of the Department of Environment, Climate Change and Water. Aerial photograph by Andrew Brown Photography. Photograph of Aragunnu Beach by Preston Cope, NPWS. Inquiries about this park or this plan of management should be directed to the Ranger at the NPWS Narooma Office, PO Box 282, Narooma, 2546 or by telephone on (02) 4476 0800. © Department of Environment, Climate Change and Water (NSW) 2011. Use permitted with appropriate acknowledgment. ISBN 978 1 74293 219 4 DECCW 2011/0246 FOREWORD Mimosa Rocks National Park covers 5,804 hectares between Tathra and Bermagui on the Far South Coast of NSW. It includes 20 kilometres coastline as well as areas of hinterland forest. The park conserves three endangered ecological communities, including an area of rainforest at Bunga Head, and provides habitat for three endangered bird species and for 19 vulnerable animals. It also contains fossils from the Devonian period and most of the catchment of Nelson Creek, which is the principal tributary of Nelson Lagoon. Mimosa Rocks National Park also contains places of cultural significance to local Aboriginal people, and evidence of past pastoral, timber harvesting, gold mining and recreational ventures. -

Tocal - the Changing Moods of a Rural Estate", Provided Access to Her Historical Research Files and Offered Helpful Information
T 0 C A L DRAFT CONSERVATION PLAN VOLUME 1 CONSERVATION PLAN JULY 1987 1111 PREPARED BY: Philip Cox, Richardson, Taylor & Partners FOR: C.B. Alexander Foundation N.S.W. Department of Agriculture T 0 C A L DRAFT CONSERVATION PLAN VOLUME 1 CONSERVATION PLAN JULY 1987 HI PREPARED BY: Philip Cox, Richardson, Taylor & Partners FOR: C.B. Alexander Foundation N.S.W. Department of Agriculture ACKNOWLEDGEHENTS The assistance in the preparation of this report has been greatly appreciated especially: Judy White - who permitted the direct use of her book "Tocal - The Changing Moods of a Rural Estate", provided access to her historical research files and offered helpful information. Dr John Drinan and Cameron Archer of the C.B. Alexander Agricultural College for advice on, and assistance in accessing information from College records. Nancy Reynolds for her recollections of Tocal from the 1920's. Bob Donaldson of the University of Newcastle who provided the measured drawings of many of the buildings and granted permission to use them in the report. CONTENTS 1.0 INTRODUCTION 1.1 Introduction 1.2 The Brief 1.3 The C.B. Alexander Foundation 1.4 The College 1.5 The Tocal Property 1.6 Status of Tocal 2.0 HISTORICAL DEVELOPMENT 2.1 History of Tocal 2.2 Outline History 2.3 A brief history of the Structures 2.4 Summary Table of Structures 2.5 Other elements '2.6 The People 2.7 Present Condition 3.0 ANALYSIS 3.1 Introduction 3.2 Comparative Study 3.3 The Property 3.4 The Structures 4.0 STATEMENT OF SIGNIFICANCE 4.1 Introduction 4.2 Assessment Table 4.3 Statement -

Bega Valley Local Government Area
Funded by the NSW Government under the NSW Water Safety watersafety.nsw.gov.au Black Spots Fund A NSW Government water safety initiative Coastal Public Safety Risk Assessment Bega Valley Local Government Area June 2014 Coastal Public Safety Risk Assessment: Bega Valley Local Government Area © Surf Life Saving New South Wales, Belrose 2014 All Rights Reserved. Disclaimer This work is copyright. However, material form this publication may be copied or published by State Government agencies without the permission of SLSNSW on the condition that the meaning of the material is not altered and SLSNSW is acknowledged as the source of the material. Any other persons or bodies wishing to use the material must seek permission. The views expressed herein do not necessarily reflect the views of the NSW Government. Page ii of 136 Coastal Public Safety Risk Assessment: Bega Valley Local Government Area TABLE OF CONTENTS EXECUTIVE SUMMARY .................................................................................................................................. VII 1 INTRODUCTION, SCOPE & CONTEXT ......................................................................................................... 6 1.1 INTRODUCTION ........................................................................................................................................... 6 1.2 CONTEXT AND SCOPE ................................................................................................................................... 8 1.3 LIMITATIONS .............................................................................................................................................. -

Handbook, 1978
THE UNIVERSITY OF MELBOURNE FACULTY 0F ARTS HANDBOOK, 1978 PUBLISHED BY THE UNIVERSITY ADDENDUM (This paragraph to be read together with page 71.) STUDENT WORK LOAD Students will note that the Handbook specifies hours for prescribed lectures and classes. In all departments, essays and reading guides impose extra load on student time. Supervision and correction of essays and guidance in reading are the responsibility of staff and occasion many hours of individual consultation between students and staff. Students should be aware that a minimum of 8 hours per week per subject will need to be spent in these required activities, in addition to the formal contact hours specified in the details for each subject. In exceptional circumstances the Council is empowered to suspend subjects and to vary the syllabus of a subject. Details of any such alteration will be available from the appropriate Faculty or Board of Studies and will be announced on departmental notice-boards. SCIENCE SUBJECTS — ENROLMENT PROCEDURE Students enrolling for any subjects in Computer Science, Mathematics, Statistics or any other subject listed under the heading 'Science Subjects' In section 'Details of Subjects' should consult the Faculty of Science Handbook for correct subject and unit numbers; and should also consult the Assistant to the Dean of Science. TABLE OF CONTENTS Officers of the Faculty of Arts 6 Directory 7 Senior Teaching Staff 9 General Information 15 Student Information Booklet 1978 15 Dates in 1978 15 Enrolment 15 Location of Lectures and Tutorials 16 Part-time Students 16 Evening Lectures 16 Leave of Absence 16 University General Principles of Selection for First-Year Courses 17 Selection Into Arts 19 Application Procedures for New Students (including Graduates) 19 Transfers from other faculties 19 Students wishing to resume an Arts Course 19 Special Principles of Selection in Faculty of Arts 19 Undergraduate Quota 19 Sub-quotas 20 Subject Quotas 21 Reservations of Places in B.A. -

How to Kill a Town Without Really Trying
Circulation 2000 plus online visits Issue No 109 June 2012 How to kill a town without really trying The media is full of stories of small Government passed new anti-discrimination guidelines but I have cut and pasted just two Australian towns struggling to maintain their laws: examples: businesses and populations. In our own area The Disability (Access to Premises - Limited application to new parts and there are more small towns or villages than buildings) Standards 2010 (the Premises ‘affected part’ in existing buildings there are large towns and they are struggling. Standards) commence on 1 May 2011. Any When new building work takes place in If you live in one of these small towns you application for a building approval for a new an existing building and a building approval become aware of the diversity of people and the building or upgrade of an existing building on is required for that new work, the requirements entrepreneurial nature of a lot of the residents. or after that date will trigger the application for upgrading access are limited to the area Ideas abound, none of them grandiose – just a of the Premises Standards. These standards of new work and the ‘affected part’. Access small business, supposedly the backbone of the stipulate that toilet facilities are to be access requirements are not imposed outside the area Australian economy, that someone thinks is a friendly for disabled people. of the new work. good idea and may just make them a living and However, and this is where communication Toilet concession add another attraction to the small town. -

The West New Guinea Debacle
The West New Guinea Debacle: Dutch Decolonisation and Indonesia, 1945-1962 To my daughter Stephanie Stewart and her husband Anthony in appreciation of your caring support of Christine and Daniel. Cover photo: Sukarno, holding a hoe (pacol), poses as the ‘first farmer’ of the nation. Photo courtesy of Greg Poulgrain. The West New Guinea Debacle: Dutch Decolonisation and Indonesia, 1945-1962 C.L.M. Penders Crawford House Publishing ADELAIDE A CHP Production Produced and published in Australia by Crawford House Publishing Pty Ltd P.O. Box 181 Hindmarsh SA 5007 Tel: +61 8 8340 1411 Fax: +61 8 8340 1811 Email: [email protected] Web: http://www. chp.com.au Published in North America by University of Hawai’i Press 2840 Kolowalu Street Honolulu, Hawai’i 96822 Published in Europe by KITLV Press Koninklijk Instituut voor Taal-, Land- en Volkenkunde (Royal Institute of Linguistics and Anthropology) P.O. Box 9515, 2300 RA Leiden, The Netherlands © 2002 C.L.M. Penders National Library of Australia Cataloguing-in-Publication entry Penders, C.L.M. (Christiaan Lambert Maria), 1928–. The West New Guinea Debacle: Dutch decolonisation and Indonesia, 1945-1962. Bibliography. Includes index. ISBN 1 86333 205 7. 995.1035 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying or otherwise, without the prior permission of the publishers. Printed in India by Thomson Press 00 01 02 03 04 5 4 3 2 1 CONTENTS Acknowledgements viii Map x I