Molecular Evolution of Color Vision in Vertebrates
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

General Introduction to the Chemistry of Dyes
GENERAL INTRODUCTION TO THE CHEMISTRY OF DYES 1. Principles of Colour Chemistry 1.1 Basis for colour Unlike most organic compounds, dyes possess colour because they 1) absorb light in the visible spectrum (400–700 nm), 2) have at least one chromophore (colour-bearing group), 3) have a conjugated system, i.e. a structure with alternating double and single bonds, and 4) exhibit resonance of electrons, which is a stabilizing force in organic compounds (Abrahart, 1977). When any one of these features is lacking from the molecular structure the colour is lost. In addition to chromophores, most dyes also contain groups known as auxochromes (colour helpers), examples of which are carboxylic acid, sulfonic acid, amino, and hydroxyl groups. While these are not responsible for colour, their presence can shift the colour of a colourant and they are most often used to influence dye solubility. Figure 1 shows the relationships between wavelength of visible and colour absorbed/observed. Other factors contributing to colour are illustrated in Figures 2–4. Fig. 1. Wavelength of light absorption versus colour in organic dyes Wavelength Absorbed (nm) Colour Absorbed Colour Observed 400–435 Violet Yellow-Green 435–480 Blue Yellow 480–490 Green-Blue Orange 490–500 Blue-Green Red 500–560 Green Purple 560–580 Yellow-Green Violet 580–595 Yellow Blue 595–605 Orange Green-Blue 605–700 Red Blue-Green –55– 56 IARC MONOGRAPHS VOLUME 99 Fig. 2. Examples of chromophoric groups present in organic dyes O O N N N N O N N O H N Anthraquinone H N Nitro Azo N N Ar C N N C Ar Methine Phthalocyanine Triarylmethane Fig. -

Identification and Distribution of Dietary Precursors of The
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Vision Research 39 (1999) 219–229 Identification and distribution of dietary precursors of the Drosophila visual pigment chromophore: analysis of carotenoids in wild type and ninaD mutants by HPLC David R. Giovannucci *, Robert S. Stephenson Department of Biological Sciences, Wayne State Uni6ersity, Detroit, MI 48202, USA Received 3 July 1997; received in revised form 10 November 1997 Abstract A dietary source of retinoid or carotenoid has been shown to be necessary for the biosynthesis of functional visual pigment in flies. In the present study, the larvae or adults of Drosophila melanogaster were administered specific carotenoid-containing diets and high performance liquid chromatography was used to identify and quantify the carotenoids in extracts of wild type and ninaD visual mutant flies. When b-carotene was fed to larvae, wild type flies were shown to hydroxylate this molecule and to accumulate zeaxanthin and a small amount of b-cryptoxanthin. Zeaxanthin content was found to increase throughout development and was a major carotenoid peak detected in the adult fly. Carotenoids were twice as effective at mediating zeaxanthin accumulation when provided to larvae versus adults. In the ninaD mutant, zeaxanthin content was shown to be specifically and significantly altered compared to wild type, and was ineffective at mediating visual pigment synthesis when provided to both larval and adult mutant flies. It is proposed that zeaxanthin is the larval storage form for subsequent visual pigment chromophore biosynthesis during pupation, that zeaxanthin or b-crytoxanthin is the immediate precursor for light-independent chromophore synthesis in the adult, and that the ninaD mutant is defective in this pathway. -

The Complexity and Origins of the Human Eye: a Brief Study on the Anatomy, Physiology, and Origin of the Eye
Running Head: THE COMPLEX HUMAN EYE 1 The Complexity and Origins of the Human Eye: A Brief Study on the Anatomy, Physiology, and Origin of the Eye Evan Sebastian A Senior Thesis submitted in partial fulfillment of the requirements for graduation in the Honors Program Liberty University Spring 2010 THE COMPLEX HUMAN EYE 2 Acceptance of Senior Honors Thesis This Senior Honors Thesis is accepted in partial fulfillment of the requirements for graduation from the Honors Program of Liberty University. ______________________________ David A. Titcomb, PT, DPT Thesis Chair ______________________________ David DeWitt, Ph.D. Committee Member ______________________________ Garth McGibbon, M.S. Committee Member ______________________________ Marilyn Gadomski, Ph.D. Assistant Honors Director ______________________________ Date THE COMPLEX HUMAN EYE 3 Abstract The human eye has been the cause of much controversy in regards to its complexity and how the human eye came to be. Through following and discussing the anatomical and physiological functions of the eye, a better understanding of the argument of origins can be seen. The anatomy of the human eye and its many functions are clearly seen, through its complexity. When observing the intricacy of vision and all of the different aspects and connections, it does seem that the human eye is a miracle, no matter its origins. Major biological functions and processes occurring in the retina show the intensity of the eye’s intricacy. After viewing the eye and reviewing its anatomical and physiological domain, arguments regarding its origins are more clearly seen and understood. Evolutionary theory, in terms of Darwin’s thoughts, theorized fossilization of animals, computer simulations of eye evolution, and new research on supposed prior genes occurring in lower life forms leading to human life. -

Emanuele Serrelli Nathalie Gontier Editors Explanation, Interpretation
Interdisciplinary Evolution Research 2 Emanuele Serrelli Nathalie Gontier Editors Macroevolution Explanation, Interpretation and Evidence Interdisciplinary Evolution Research Volume 2 Series editors Nathalie Gontier, Lisbon, Portugal Olga Pombo, Lisbon, Portugal [email protected] About the Series The time when only biologists studied evolution has long since passed. Accepting evolution requires us to come to terms with the fact that everything that exists must be the outcome of evolutionary processes. Today, a wide variety of academic disciplines are therefore confronted with evolutionary problems, ranging from physics and medicine, to linguistics, anthropology and sociology. Solving evolutionary problems also necessitates an inter- and transdisciplinary approach, which is why the Modern Synthesis is currently extended to include drift theory, symbiogenesis, lateral gene transfer, hybridization, epigenetics and punctuated equilibria theory. The series Interdisciplinary Evolution Research aims to provide a scholarly platform for the growing demand to examine specific evolutionary problems from the perspectives of multiple disciplines. It does not adhere to one specific academic field, one specific school of thought, or one specific evolutionary theory. Rather, books in the series thematically analyze how a variety of evolutionary fields and evolutionary theories provide insights into specific, well-defined evolutionary problems of life and the socio-cultural domain. Editors-in-chief of the series are Nathalie Gontier and Olga Pombo. The -

Ba3(P1−Xmnxo4)2 : Blue/Green Inorganic Materials Based on Tetrahedral Mn(V)
Bull. Mater. Sci., Vol. 34, No. 6, October 2011, pp. 1257–1262. c Indian Academy of Sciences. Ba3(P1−xMnxO4)2 : Blue/green inorganic materials based on tetrahedral Mn(V) SOURAV LAHA, ROHIT SHARMA, S V BHAT†, MLPREDDY‡, J GOPALAKRISHNAN∗ and S NATARAJAN Solid State and Structural Chemistry Unit, †Department of Physics, Indian Institute of Science, Bangalore 560 012, India ‡Chemical Science and Technology Division, National Institute for Interdisciplinary Science and Technology (NIIST), Thiruvananthapuram 695 019, India MS received 11 May 2011 ) 3− 2 Abstract. We describe a blue/green inorganic material, Ba3(P1−xMnxO4 2 (I) based on tetrahedral MnO4 :3d chromophore. The solid solutions (I) which are sky-blue and turquoise-blue for x ≤ 0·25 and dark green for x ≥ 0·50, 3− are readily synthesized in air from commonly available starting materials, stabilizing the MnO4 chromophore in an isostructural phosphate host. We suggest that the covalency/ionicity of P–O/Mn–O bonds in the solid solutions tunes the crystal field strength around Mn(V) such that a blue colour results for materials with small values of x. The material could serve as a nontoxic blue/green inorganic pigment. Keywords. Blue/green inorganic material; barium phosphate/manganate(V); tetrahedral manganate(V); blue/green chromophore; ligand field tuning of colour. 1. Introduction field transitions within an unusual five coordinated trigo- nal bipyramidal Mn(III). A turquoise-blue solid based on Inorganic solids displaying bright colours are important Li1·33Ti1·66O4 spinel oxide wherein the colour arises from as pigment materials which find a wide range of appli- an intervalence charge-transfer between Ti3+ and Ti4+ has cations in paints, inks, plastics, rubbers, ceramics, ena- also been described recently (Fernández-Osorio et al 2011). -
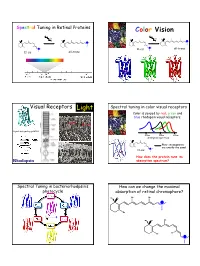
Color Vision Light
Spectral Tuning in Retinal Proteins Color Vision hν hν N N H H H H N all-trans N 11-cis 11-cis all-trans Visual Receptors Light Spectral tuning in color visual receptors Color is sensed by red, green and blue rhodopsin visual receptors. Rod Cone G-protein signaling pathway 400nm 500nm 600nm absorption spectrum Their chromophores H are exactly the same! 11-cis N How does the protein tune its Rhodopsin absorption spectrum? Spectral Tuning in bacteriorhodpsin’s How can we change the maximal photocycle absorption of retinal chromophore? Me Me Me Me N H Me Me Me Me N H Me Me Me Me Me Me Me H N 1 Excitation energy determines the Electronic Absorption maximal absorption Me Me Me Me π π* N - H S1 Me Absorption of light in the UV-VIS region of the S0 spectrum is due to Response excitation of electrons to higher energy levels. Me Me Me 13 7911 15 N H Me Me π-π* excitation in polyenes π-π* excitation in polyenes π∗ π∗ π∗ π∗ ∆E π∗ E photon E π π π π π Ground state (S0) Excited state (S1) ∆E (excitation energy, band gap) = hν = hc/λ blue-shift red-shift π-π* excitation in polyenes Tuning the length of the conjugated backbone β-carotene O O Vitamin A2 (retinal II) Vitamin A1 (retinal I) Longer wavelength Short wavelength 2 Retinal I Retinal II OPSIN SHIFT: how protein tunes the absorption maximum of its chromophore. Maximal absorption of protonated retinal Schiff base in: Water/methanol solution: 440 nm bR: 568 nm rod Rh: 500 nm red receptor: 560 nm green receptor: 530 nm blue receptor: 426 nm Salmon: different retinals in different stages of life Electrostatics and opsin shift Electrostatics and opsin shift S S positive charge 2 positive charge 2 S2 S2 Me Me Me Me Me Me S1 S1 S1 S1 + + + + N N H H Me O S0 Me O S0 Me O Me O C C Asp (Glu) Asp (Glu) S0 S0 no protein in protein no protein in protein counterion counterion • The counterion stabilizes the positive charge of the chromophore. -

New Perspectives on Eye Development and the Evolution of Eyes and Photoreceptors
Journal of Heredity 2005:96(3):171–184 ª 2005 The American Genetic Association doi:10.1093/jhered/esi027 Advance Access publication January 13, 2005 THE WILHEMINE E. KEY 2004 INVITATIONAL LECTURE New Perspectives on Eye Development and the Evolution of Eyes and Photoreceptors W. J. GEHRING From the Department of Cell Biology, Biozentrum, University of Basel, Klingelbergstrasse 70, 4056 Basel, Switzerland Address correspondence to Walter Gehring at the address above, or e-mail: [email protected] Walter J. Gehring is Professor at the Biozentrum of the University of Basel, Switzerland. He obtained his Ph.D. at the University of Zurich in 1965 and after two years as a research assistant of Professor Ernst Hadorn he joined Professor Alan Garen’s group at Yale University in New Haven as a postdoctoral fellow. In 1969 he was appointed as an associate professor at the Yale Medical School and 1972 he returned to Switzerland to become a professor of developmental biology and genetics at the Biozentrum of the University of Basel. He has served as Secretary General of the European Molecular Biology Organization and President of the International Society for Developmental Biologists. He was elected as a Foreign Associate of the US National Academy of Sciences, the Royal Swedish Academy of Science, the Leopoldina, a Foreign Member of the Royal Society of London for Improving Natural Knowledge and the French Acade´mie des Sciences. Walter Gehring has been involved in studies of Drosophila genetics and development, particularly in the analysis of cell determination in the embryo and transdetermination of imaginal discs. -

Lynn Margulis and Dorion Sagan, Acquiring Genomes
CONTENTS Forewordby Ernst Mayr XI xv CaJ1•1thtO JOOJ by lfnn M.,1i1ll1111J l>nrlun S1tc11n Preface Pulttl1h,Jby 1111k Rook,, PART ONE. THE EVOLUTIONARY IMPERATIVE AM,mber of rh, l'wucu1Book, Group. 1 Darwinism Not Neodarwinism 3 Allrlahu re1crved. Printed in the United States of America. No part of this book may be 2 Darwin's Dilemma 25 rc~r<>ducedin any manner whatsoever without written permission except in the case of 3 Relative Individuality 51 briefquotations embodied in critical articles and reviews.For information, address Basic 67 Books, 387 Park Avenue South, New York NY 10016-8810. 4 The Natural Selector 5 Principles of Evolutionary Novelty 71 Library of Congress Cataloging-in-Publication Data PART TWO. THE MICROBE IN EVOLUTION Margulis, Lynn, 1938- Acquiring genomes : a theory of the origins of species / Lynn Margulis and Dorion 6 Species and Cells 81 Sagan.-lst ed. 7 History of the Heritable 89 p. cm. Includes bibiliographical references PART THREE. PLANETARY LEGACY ISBN 0-465-04391-7 (hardcover) 1. Species. 2. Symbiogenesis. 3. Evolution (Biology). 4. Sagan Dorion 1959- II 123 Title. ' ' . 8 Gaian Planet 139 QH380 .M37 2002 9 Eukaryosis in an Anoxic World 576.8'6-dc21 2002001521 PART FOUR. CONSORTIA 165 Text design by TrishWilkimon 10 Seaworthy Alliances Set in 12.5-point AGaramond by The Perseus Books Group 11 Plant Proclivities 185 12 Chromosome Dance: The Fission Theory 191 FIRST EDITION 13 Darwin Revisited: 02 03 04 05 / IO 9 8 7 6 5 4 3 2 1 Spedes in the Evolutionary Dialogue 201 ..•,, •HI /,,/tit,,,,,,/ 1//11,11,111,,,,, -

Indigo Chromophores and Pigments Structure and Dynamics
Dyes and Pigments 172 (2020) 107761 Contents lists available at ScienceDirect Dyes and Pigments journal homepage: www.elsevier.com/locate/dyepig Indigo chromophores and pigments: Structure and dynamics T ∗ V.V. Volkova, R. Chellib, R. Righinic, C.C. Perrya, a Interdisciplinary Biomedical Research Centre, School of Science and Technology, Nottingham Trent University, Clifton Lane, Nottingham, NG11 8NS, United Kingdom b Dipartimento di Chimica, Università degli Studi di Firenze, Via della Lastruccia 3, Sesto Fiorentino, 50019, Italy c European Laboratory for Non-Linear Spectroscopy (LENS), Università degli Studi di Firenze, Via Nello Carrara 1, Sesto Fiorentino, 50019, Italy ARTICLE INFO ABSTRACT Keywords: In this study, we explore the molecular mechanisms of the stability of indigo chromophores and pigments. Indigo Assisted with density functional theory, we compare visible, infrared and Raman spectral properties of model Raman molecules, chromophores and pigments derived from living organisms. Using indigo carmine as a representative Infrared model system, we characterize the structure and dynamics of the chromophore in the first electronic excited Density functional theory state using femtosecond visible pump-infrared probe spectroscopy. Results of experiments and theoretical stu- Excited state dies indicate that, while the trans geometry is strongly dominant in the electronic ground state, upon photo- excitation, in the Franck-Condon region, some molecules may experience isomerization and proton transfer dynamics. If this happens, however, the normal modes of the trans geometry of the electronic excited state are reconfirmed within several hundred femtoseconds. Supported by quantum theory, first, we ascribe stabilization of the trans geometry in the Franck-Condon region to the reactive character of the potential energy surface for the indigo chromophore when under the cis geometry in the electronic excited state. -

Chromophore of an Enhanced Green Fluorescent Protein Can Play a Photoprotective Role Due to Photobleaching
International Journal of Molecular Sciences Article Chromophore of an Enhanced Green Fluorescent Protein Can Play a Photoprotective Role Due to Photobleaching Joanna Krasowska 1 , Katarzyna Pierzchała 2,3, Agnieszka Bzowska 1 ,László Forró 3 , Andrzej Sienkiewicz 3,4,5,* and Beata Wielgus-Kutrowska 1,* 1 Division of Biophysics, Institute of Experimental Physics, Faculty of Physics, University of Warsaw, Pasteura 5, 02-093 Warsaw, Poland; [email protected] (J.K.); [email protected] (A.B.) 2 Laboratory for Functional and Metabolic Imaging (LIFMET), Institute of Physics (IPHYS), School of Basic Sciences (SB), Ecole Polytechnique Fédérale de Lausanne (EPFL), CH-1015 Lausanne, Switzerland; katarzyna.pierzchala@epfl.ch 3 Laboratory of Physics of Complex Matter (LPMC), Institute of Physics (IPHYS), School of Basic Sciences (SB), Ecole Polytechnique Fédérale de Lausanne (EPFL), CH-1015 Lausanne, Switzerland; laszlo.forro@epfl.ch 4 Laboratory for Quantum Magnetism (LQM), Institute of Physics (IPHYS), School of Basic Sciences (SB), École Polytechnique Fédérale de Lausanne (EPFL), Station 3, CH-1015 Lausanne, Switzerland 5 ADSresonances, Route de Genève 60B, CH-1028 Préverenges, Switzerland * Correspondence: andrzej.sienkiewicz@epfl.ch (A.S.); [email protected] (B.W.-K.) Abstract: Under stress conditions, elevated levels of cellular reactive oxygen species (ROS) may impair crucial cellular structures. To counteract the resulting oxidative damage, living cells are equipped with several defense mechanisms, including photoprotective functions of specific proteins. Here, we discuss the plausible ROS scavenging mechanisms by the enhanced green fluorescent Citation: Krasowska, J.; Pierzchała, protein, EGFP. To check if this protein could fulfill a photoprotective function, we employed electron K.; Bzowska, A.; Forró, L.; Sienkiewicz, A.; Wielgus-Kutrowska, spin resonance (ESR) in combination with spin-trapping. -

Evolutionary Theory: the Basics
Evolutionary Theory: The Basics Cogs 184 * Modeling Cognitive Evolution When constructing an evolutionary scenario… …need to understand, apply Biological Principles! Some Basic Concepts • Geneotype • The organism's genetic makeup • Mostly recipes for building proteins • Alleles (versions) of a gene can be dom/recessive • Phenotype • The organism's physical & behavioral characteristics Some Basic Concepts • Most phenotypic traits are polygenetic • e.g. Even eye color requires 6 genes to code for • So how silly is it to discuss/seek "the language gene" FoxP2 Gene Some Basic Concepts • Most phenotypic traits are polygenetic • e.g. Even eye color requires 6 genes to code for • So how silly is it to discuss/seek "the language gene" • Altho sometimes one small genetic change >> huge phenotypic effects • A change in a CONTROL gene (Operator, Supressor) • Can alter timing, order of processes • e.g. During brain development, cells first duplicate, then differentiate • By suppressing onset of differentiation, duplication continues longer >> can triple brain size! Some Basic Concepts • Because genetic material not generally available in fossils... • Although note recent Neanderthal discovery! • We will mainly use phenotypic traits as the basis for our evolutionary scenarios • Note: This will mean ASSUMING those traits are HERETABLE!! • "Heretiability" mainly genetic • We will later also discuss MEMES passed to next generation • Meme = cultural unit of selection • Religious practice • Writing • Democracy, etc. etc. Evolution by Natural Selection Charles Darwin Evolution by Natural Selection 1) Variability, across a population, in a heritable trait • Some sources of variance: • Recombination, Mutation (e.g. Insertion, Translocation), etc. Evolution by Natural Selection 1) Variability, across a population, in a heritable trait • Some sources of variance: • Recombination, Mutation (e.g. -

THE FLUORESCENCE from the CHROMOPHORE of the PURPLE MEMBRANE PROTEIN Rajni GOVINDJEE, BRIAN BECHER, and THOMAS G
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector THE FLUORESCENCE FROM THE CHROMOPHORE OF THE PURPLE MEMBRANE PROTEIN RAJNi GOVINDJEE, BRIAN BECHER, AND THOMAS G. EBREY, Department ofPhysiology and Biophysics, University ofIllinois, Urbana, Illinois 61801 U.S.A. ABSTRACr The fluorescence from the purple membrane protein (PM) of Halo- bacterium halobium and its relation to the primary photochemical events have been studied. The emission spectrum at 770K has structure, with peaks at 680, 710-715, and 730-735 nm. The excitation spectrum shows a single peak centered at 580 nm. This and a comparison of the fluorescence intensity at 770K under a variety of con- ditions with the amounts of the bathoproduct (or K, the only photoproduct seen at this temperature) formed suggest that the source of the fluorescence is the purple mem- brane itself, not the photoproduct. From the difference in several of their properties, we suggest that the fluorescing state of the pigment is different from the excited state which leads to photoconversion. Light energy absorbed by the purple membrane (PM) of Halobacterium halobium (bacteriorhodopsin) can be converted into chemical free energy of a proton gradient across the cell membrane (Oesterhelt and Stoeckenius, 1973). This pumping of pro- tons across the membrane is associated with the formation and decay of a series of spectroscopic intermediates, the first of which is formed photochemically. The follow- ing is a schematic representation of the cyclic reaction of PM568 (Lozier et al., 1975): PM568 i K628 L M412 --N --0 t hp I In the primary photochemical event, PM hp K, the photon's energy is used to con- vert PM into a high free-energy species, K, whose free energy is used to drive the rest of the cycle (Rosenfeld et al., 1977).