Color in Commercial Glasses
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

General Introduction to the Chemistry of Dyes
GENERAL INTRODUCTION TO THE CHEMISTRY OF DYES 1. Principles of Colour Chemistry 1.1 Basis for colour Unlike most organic compounds, dyes possess colour because they 1) absorb light in the visible spectrum (400–700 nm), 2) have at least one chromophore (colour-bearing group), 3) have a conjugated system, i.e. a structure with alternating double and single bonds, and 4) exhibit resonance of electrons, which is a stabilizing force in organic compounds (Abrahart, 1977). When any one of these features is lacking from the molecular structure the colour is lost. In addition to chromophores, most dyes also contain groups known as auxochromes (colour helpers), examples of which are carboxylic acid, sulfonic acid, amino, and hydroxyl groups. While these are not responsible for colour, their presence can shift the colour of a colourant and they are most often used to influence dye solubility. Figure 1 shows the relationships between wavelength of visible and colour absorbed/observed. Other factors contributing to colour are illustrated in Figures 2–4. Fig. 1. Wavelength of light absorption versus colour in organic dyes Wavelength Absorbed (nm) Colour Absorbed Colour Observed 400–435 Violet Yellow-Green 435–480 Blue Yellow 480–490 Green-Blue Orange 490–500 Blue-Green Red 500–560 Green Purple 560–580 Yellow-Green Violet 580–595 Yellow Blue 595–605 Orange Green-Blue 605–700 Red Blue-Green –55– 56 IARC MONOGRAPHS VOLUME 99 Fig. 2. Examples of chromophoric groups present in organic dyes O O N N N N O N N O H N Anthraquinone H N Nitro Azo N N Ar C N N C Ar Methine Phthalocyanine Triarylmethane Fig. -

Quarterly Journal of the All India Glass Manufacturers' Federation
Vol. 3 | No. 4 | January - March 2016 Quarterly Journal of The All India Glass Manufacturers’ Federation Bi-lingual www.aigmf.com Technical Articles Prof. (Dr.) A. K. Bandyopadhyay Prof. (Dr.) A Sustainable 50 for postage postage for 50 ` ASS ASS www.aigmf.com Building and Packaging material An Publication - GlASS Gl Gl 500 (within India) + + India) (within 500 ` ` Kanch | Vol. 3 | No. 4 | January-March 2016 2 Overseas: US$ 60 (including postage and bank charges) bank and postage (including 60 US$ Overseas: Order Print Copies: Print Order Price: Price: www.aigmf.com President SANJAY GANJOO Sr. Vice President ARUN KUMAR DUKKIPATI Vice President RAJ KUMAR MITTAL Hon. General Secretary BHARAT SOMANY Hon. Treasurer SANJAY AGARWAL Member Editorial Board A K Bandyopadhyay Quarterly Journal of THE ALL INDIA GLASS MANUFACTURERS’ FEDERATION Former Principal, Govt. College of Engineering & Ceramic Technology-GCECT, Kolkata DEVENDRA KUMAR Prof. & HOD, Dept. of Ceramic, Indian Institute of Technology (Banaras Hindu University) Vol. 3 | No. 4 | January-March 2016 K K SHARMA President, NIGMA and Plant Head, HNG Neemrana, Rajasthan MEMBER ASSOCIatIONS EASTERN INDIA GLASS MANUFACTURERS’ ASSOCIATION (EIGMA) Contents c/o La Opala RG Ltd. Chitrakoot, 10th Floor, 230 A, A.J.C. Bose Road From President's Desk 5 Kolkata - 700 020 President - Sushil Jhunjhunwala Glass as Vital Building Material for Smart / Solar Cities NORTHERN INDIA GLASS MANUFACTURERS’ ASSOCIATION (NIGMA) & c/o Hindustan National Glass & Industries Limited 6 Post Office - Bahadurgarh, Jhajjar, Haryana-124 507 Book Launch: “Glass - A Sustainable Building and Packaging President - KK Sharma Material” Vice President - Jimmy Tyagi Honorary General Secretary - NN Goyal Glass News 13 Secretary & Treasurer - JB Bhardwaj SOUTH INDIA GLASS MANUFACTURERS’ ASSOCIATION (SIGMA) Smart City and Glasses for Flat-Screen Products – Part II 21 c/o AGI Glasspac (An SBU of HSIL Ltd.) Glass Factory Road, Off. -

Identification and Distribution of Dietary Precursors of The
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Vision Research 39 (1999) 219–229 Identification and distribution of dietary precursors of the Drosophila visual pigment chromophore: analysis of carotenoids in wild type and ninaD mutants by HPLC David R. Giovannucci *, Robert S. Stephenson Department of Biological Sciences, Wayne State Uni6ersity, Detroit, MI 48202, USA Received 3 July 1997; received in revised form 10 November 1997 Abstract A dietary source of retinoid or carotenoid has been shown to be necessary for the biosynthesis of functional visual pigment in flies. In the present study, the larvae or adults of Drosophila melanogaster were administered specific carotenoid-containing diets and high performance liquid chromatography was used to identify and quantify the carotenoids in extracts of wild type and ninaD visual mutant flies. When b-carotene was fed to larvae, wild type flies were shown to hydroxylate this molecule and to accumulate zeaxanthin and a small amount of b-cryptoxanthin. Zeaxanthin content was found to increase throughout development and was a major carotenoid peak detected in the adult fly. Carotenoids were twice as effective at mediating zeaxanthin accumulation when provided to larvae versus adults. In the ninaD mutant, zeaxanthin content was shown to be specifically and significantly altered compared to wild type, and was ineffective at mediating visual pigment synthesis when provided to both larval and adult mutant flies. It is proposed that zeaxanthin is the larval storage form for subsequent visual pigment chromophore biosynthesis during pupation, that zeaxanthin or b-crytoxanthin is the immediate precursor for light-independent chromophore synthesis in the adult, and that the ninaD mutant is defective in this pathway. -

Ba3(P1−Xmnxo4)2 : Blue/Green Inorganic Materials Based on Tetrahedral Mn(V)
Bull. Mater. Sci., Vol. 34, No. 6, October 2011, pp. 1257–1262. c Indian Academy of Sciences. Ba3(P1−xMnxO4)2 : Blue/green inorganic materials based on tetrahedral Mn(V) SOURAV LAHA, ROHIT SHARMA, S V BHAT†, MLPREDDY‡, J GOPALAKRISHNAN∗ and S NATARAJAN Solid State and Structural Chemistry Unit, †Department of Physics, Indian Institute of Science, Bangalore 560 012, India ‡Chemical Science and Technology Division, National Institute for Interdisciplinary Science and Technology (NIIST), Thiruvananthapuram 695 019, India MS received 11 May 2011 ) 3− 2 Abstract. We describe a blue/green inorganic material, Ba3(P1−xMnxO4 2 (I) based on tetrahedral MnO4 :3d chromophore. The solid solutions (I) which are sky-blue and turquoise-blue for x ≤ 0·25 and dark green for x ≥ 0·50, 3− are readily synthesized in air from commonly available starting materials, stabilizing the MnO4 chromophore in an isostructural phosphate host. We suggest that the covalency/ionicity of P–O/Mn–O bonds in the solid solutions tunes the crystal field strength around Mn(V) such that a blue colour results for materials with small values of x. The material could serve as a nontoxic blue/green inorganic pigment. Keywords. Blue/green inorganic material; barium phosphate/manganate(V); tetrahedral manganate(V); blue/green chromophore; ligand field tuning of colour. 1. Introduction field transitions within an unusual five coordinated trigo- nal bipyramidal Mn(III). A turquoise-blue solid based on Inorganic solids displaying bright colours are important Li1·33Ti1·66O4 spinel oxide wherein the colour arises from as pigment materials which find a wide range of appli- an intervalence charge-transfer between Ti3+ and Ti4+ has cations in paints, inks, plastics, rubbers, ceramics, ena- also been described recently (Fernández-Osorio et al 2011). -

Float Glass Inspection and Measurement Phone: +1-651-730-4090 Fax: +1-651-730-1955 for Highest Quality and Optimized Yields
Germany Phone: +49-89-85695-0 Fax: +49-89-85695-200 USA Float Glass Inspection and Measurement Phone: +1-651-730-4090 Fax: +1-651-730-1955 for Highest Quality and Optimized Yields Korea Phone: +82-2-527-1633 Fax: +82-2-527-1635 Taiwan Phone: +886-2-2920-7899 Fax: +886-2-2920-8198 Dr. Schenk’s production site Hong Kong Phone: +852-2425-1860 Fax: +852-2425-6775 China-Beijing Dr. Schenk GmbH, established in 1985, is an innovative Phone: +86-10-6503-2159 high-tech company based near Munich, Germany. Fax: +86-10-6503-2161 Dr. Schenk develops, produces and markets optical surface inspection and measurement solutions for automated China-Shanghai quality assurance and production process monitoring. Phone: +86-21-5836-6700 The systems are a key success factor in the making and Fax: +86-21-5836-6701 converting of many materials, e.g. plastics, glass, metal, PV modules, wovens & nonwovens, and the semiconduc- tor industry. Throughout the world Dr. Schenk’s 220 employees con- For further regional sales & tinue to set new standards for the inspection of surfaces. service representatives please refer Over 10,000 m² of modern production and testing facili- to www.drschenk.com ties are available to research, development and production to apply cutting-edge optics and electronics to customer applications. The company’s objective is complete customer satisfaction. This is achieved through innovative and practical solutions that can be implemented into new and existing production lines. Local sales and service facilities around the world ensure fast support, technical service, training and consult- ing at any phase of a project. -
Color Vision Light
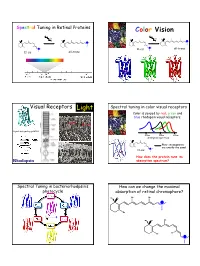
Spectral Tuning in Retinal Proteins Color Vision hν hν N N H H H H N all-trans N 11-cis 11-cis all-trans Visual Receptors Light Spectral tuning in color visual receptors Color is sensed by red, green and blue rhodopsin visual receptors. Rod Cone G-protein signaling pathway 400nm 500nm 600nm absorption spectrum Their chromophores H are exactly the same! 11-cis N How does the protein tune its Rhodopsin absorption spectrum? Spectral Tuning in bacteriorhodpsin’s How can we change the maximal photocycle absorption of retinal chromophore? Me Me Me Me N H Me Me Me Me N H Me Me Me Me Me Me Me H N 1 Excitation energy determines the Electronic Absorption maximal absorption Me Me Me Me π π* N - H S1 Me Absorption of light in the UV-VIS region of the S0 spectrum is due to Response excitation of electrons to higher energy levels. Me Me Me 13 7911 15 N H Me Me π-π* excitation in polyenes π-π* excitation in polyenes π∗ π∗ π∗ π∗ ∆E π∗ E photon E π π π π π Ground state (S0) Excited state (S1) ∆E (excitation energy, band gap) = hν = hc/λ blue-shift red-shift π-π* excitation in polyenes Tuning the length of the conjugated backbone β-carotene O O Vitamin A2 (retinal II) Vitamin A1 (retinal I) Longer wavelength Short wavelength 2 Retinal I Retinal II OPSIN SHIFT: how protein tunes the absorption maximum of its chromophore. Maximal absorption of protonated retinal Schiff base in: Water/methanol solution: 440 nm bR: 568 nm rod Rh: 500 nm red receptor: 560 nm green receptor: 530 nm blue receptor: 426 nm Salmon: different retinals in different stages of life Electrostatics and opsin shift Electrostatics and opsin shift S S positive charge 2 positive charge 2 S2 S2 Me Me Me Me Me Me S1 S1 S1 S1 + + + + N N H H Me O S0 Me O S0 Me O Me O C C Asp (Glu) Asp (Glu) S0 S0 no protein in protein no protein in protein counterion counterion • The counterion stabilizes the positive charge of the chromophore. -

ENABLING WONDERS. INSPIRING AWE. AIS Architectural Product Profile
ENABLING WONDERS. INSPIRING AWE. AIS Architectural Product Profile 1 FROM ART TO ARCHITECTURE Architecture is an emotional experience that begins in the mind of the architect. It starts from a vision that transforms into an art. Just like any artist, the architect gets to play with various materials and ideas. Glass is the latest material that is allowing architects to interpret space in a whole new way, inspire creative designs, and create structures that reflect beauty. AIS has the knowledge, expertise, and an unmatched array of products to bring an artistic idea from a vision to a masterpiece. 2 Cummins, Pune 3 ENABLING A FUTURE THAT SEES MORE AIS is India’s leading integrated glass company. Being a leader, AIS delivers top-of-the-line products and solutions through three Strategic Business Units (SBUs) of Automotive Glass, Architectural Glass and Consumer Glass. We use our glass product portfolio – which is the biggest in the country – to meet functional needs in an aesthetic and contemporary manner. With products that provide next-generation solutions, AIS brings new ideas to life – enabling an age of ‘green buildings’ and the dawn of a truly sustainable future. Taking the versatility of glass to the next level, AIS today has unmatched glass processing capabilities, including the processing of special glass products, that enables us to meet your every need, and fulfil every requirement. And help you realise your dream house in glass. 4 ARCHITECTURAL GLASS Architectural Glass, or float glass, is manufactured by floating the molten glass on a bed of molten metal, typically tin. This method gives the glass product uniform thickness and a very flat surface. -

Indigo Chromophores and Pigments Structure and Dynamics
Dyes and Pigments 172 (2020) 107761 Contents lists available at ScienceDirect Dyes and Pigments journal homepage: www.elsevier.com/locate/dyepig Indigo chromophores and pigments: Structure and dynamics T ∗ V.V. Volkova, R. Chellib, R. Righinic, C.C. Perrya, a Interdisciplinary Biomedical Research Centre, School of Science and Technology, Nottingham Trent University, Clifton Lane, Nottingham, NG11 8NS, United Kingdom b Dipartimento di Chimica, Università degli Studi di Firenze, Via della Lastruccia 3, Sesto Fiorentino, 50019, Italy c European Laboratory for Non-Linear Spectroscopy (LENS), Università degli Studi di Firenze, Via Nello Carrara 1, Sesto Fiorentino, 50019, Italy ARTICLE INFO ABSTRACT Keywords: In this study, we explore the molecular mechanisms of the stability of indigo chromophores and pigments. Indigo Assisted with density functional theory, we compare visible, infrared and Raman spectral properties of model Raman molecules, chromophores and pigments derived from living organisms. Using indigo carmine as a representative Infrared model system, we characterize the structure and dynamics of the chromophore in the first electronic excited Density functional theory state using femtosecond visible pump-infrared probe spectroscopy. Results of experiments and theoretical stu- Excited state dies indicate that, while the trans geometry is strongly dominant in the electronic ground state, upon photo- excitation, in the Franck-Condon region, some molecules may experience isomerization and proton transfer dynamics. If this happens, however, the normal modes of the trans geometry of the electronic excited state are reconfirmed within several hundred femtoseconds. Supported by quantum theory, first, we ascribe stabilization of the trans geometry in the Franck-Condon region to the reactive character of the potential energy surface for the indigo chromophore when under the cis geometry in the electronic excited state. -

Glass Shaping
MIT 3.071 Amorphous Materials 6: Glass Shaping Juejun (JJ) Hu 1 After-class reading list Fundamentals of Inorganic Glasses Ch. 20 Introduction to Glass Science and Technology Ch. 13 2 Image @ MIT. All rights reserved. This content is excluded from our Creative Commons license. For more information, see http://ocw.mit.edu/help/faq-fair-use/. “Viscosity makes things happen essentially in slow motion. If you are trying to melt a crystalline solid (like ice or an aluminum oxide ceramic), as soon as you reach the melting point, a drop of liquid forms and falls away from the melting surface. Glass, on the other hand, … gradually transforms from a hard solid to a slowly softening liquid. This soft liquid gradually stiffens as it cools (because of its increasing viscosity), allowing glass blower time to shape and manipulate the glass.” http://madsci.org/posts/archives/2007-09/1188944613.Ph.r.html 3 Viscosity reference points Working range PGM Glass blowing Lehr annealing Fiber drawing Float glass Pitch: 2.3 × 108 h 101 103 106.6 1012 1013.5 (Pa·s) Melting Working Softening Annealing Straining point point point point (Tg) point 4 Basic properties of common silicate glasses Soda-lime Borosilicate Fused silica CTE (ppm/°C) 9.2 3.2 0.5 Working point 1005 1252 N/A (°C) Softening point 696 821 1650 (°C) Annealing point 510 560 1140 (°C) Strain point (°C) 475 510 1070 5 Flat glass manufacturing: float glass process Forming of a continuous ribbon of glass using a molten tin bath Melting and refining (homogenization and bubble removal) Float bath: glass thickness controlled by flow speed Annealing: stress release Inspection, cutting and shipping 1 2.5 12 15 10 Pa·s 10 Pa·s 10 Pa·s > 10 Pa·s © H.S. -

Molecular Evolution of Color Vision in Vertebrates
Gene 300 (2002) 69–78 www.elsevier.com/locate/gene Molecular evolution of color vision in vertebrates Shozo Yokoyama* Department of Biology, Biological Research Laboratories, Syracuse University, 130 College Place, Syracuse, NY 13244, USA Received 10 December 2001; received in revised form 4 March 2002; accepted 17 July 2002 Abstract Visual systems of vertebrates exhibit a striking level of diversity, reflecting their adaptive responses to various color environments. The photosensitive molecules, visual pigments, can be synthesized in vitro and their absorption spectra can be determined. Comparing the amino acid sequences and absorption spectra of various visual pigments, we can identify amino acid changes that have modified the absorption spectra of visual pigments. These hypotheses can then be tested using the in vitro assay. This approach has been a powerful tool in elucidating not only the molecular bases of color vision, but the processes of adaptive evolution at the molecular level. q 2002 Elsevier Science B.V. All rights reserved. Keywords: Visual pigment; Wavelength absorption; Color vision; Vertebrate 1. Introduction visual pigments, it was necessary to clone opsin genes. This was initiated in 1986 when the opsin genes were cloned Vision has profound effects on the evolution of from cow and human (Nathans and Hogness, 1983, 1984; organisms by affecting survivorship through such behaviors Nathans et al., 1986). The availability of these opsin clones as mating, foraging, and predator avoidance. The data led to the isolation of other orthologous and paralogous collected by vision scientists over the last century genes from various species. Second, in the late 1980s, it demonstrate beyond any doubt that ecology has been a became possible to express opsins in cultured cells, major factor in directing the evolution of visual systems reconstitute with 11-cis-retinal, and measure the absorption (Walls, 1942; Lythgoe, 1979; Jacobs, 1981; Bowmaker, spectra of the resulting visual pigments in vitro (Oprian 1991; Yokoyama and Yokoyama, 1996). -

Chromophore of an Enhanced Green Fluorescent Protein Can Play a Photoprotective Role Due to Photobleaching
International Journal of Molecular Sciences Article Chromophore of an Enhanced Green Fluorescent Protein Can Play a Photoprotective Role Due to Photobleaching Joanna Krasowska 1 , Katarzyna Pierzchała 2,3, Agnieszka Bzowska 1 ,László Forró 3 , Andrzej Sienkiewicz 3,4,5,* and Beata Wielgus-Kutrowska 1,* 1 Division of Biophysics, Institute of Experimental Physics, Faculty of Physics, University of Warsaw, Pasteura 5, 02-093 Warsaw, Poland; [email protected] (J.K.); [email protected] (A.B.) 2 Laboratory for Functional and Metabolic Imaging (LIFMET), Institute of Physics (IPHYS), School of Basic Sciences (SB), Ecole Polytechnique Fédérale de Lausanne (EPFL), CH-1015 Lausanne, Switzerland; katarzyna.pierzchala@epfl.ch 3 Laboratory of Physics of Complex Matter (LPMC), Institute of Physics (IPHYS), School of Basic Sciences (SB), Ecole Polytechnique Fédérale de Lausanne (EPFL), CH-1015 Lausanne, Switzerland; laszlo.forro@epfl.ch 4 Laboratory for Quantum Magnetism (LQM), Institute of Physics (IPHYS), School of Basic Sciences (SB), École Polytechnique Fédérale de Lausanne (EPFL), Station 3, CH-1015 Lausanne, Switzerland 5 ADSresonances, Route de Genève 60B, CH-1028 Préverenges, Switzerland * Correspondence: andrzej.sienkiewicz@epfl.ch (A.S.); [email protected] (B.W.-K.) Abstract: Under stress conditions, elevated levels of cellular reactive oxygen species (ROS) may impair crucial cellular structures. To counteract the resulting oxidative damage, living cells are equipped with several defense mechanisms, including photoprotective functions of specific proteins. Here, we discuss the plausible ROS scavenging mechanisms by the enhanced green fluorescent Citation: Krasowska, J.; Pierzchała, protein, EGFP. To check if this protein could fulfill a photoprotective function, we employed electron K.; Bzowska, A.; Forró, L.; Sienkiewicz, A.; Wielgus-Kutrowska, spin resonance (ESR) in combination with spin-trapping. -

Precision Sensors & Applications Glass Industry
Sensors & Applications Glass Industry More Precision Sensors and measuring systems confocalDT 2421 / 2422 Confocal chromatic sensors for for glass production distance and thickness measurements Modern glass production is increasin- One-sided thickness measurement of gly determined by maximum efficiency. transparent materials Therefore, rapid access to fundamental Synchronous 2-channel measurement process variables is required in order to with max. measuring rate ensure fast control of the process. With Best price/performance ratio in its class products such as container glass, flat glass or special glasses, tight manufac- turing tolerances must be adhered to while maintaining the shortest possible cycle times. colorCONTROL ACS Sensors for color measurement of transparent materials Due to the high degree of integration as Ideal for integration into processing lines well as the high accuracy and measure- due to high measuring rates ment speed, sensors from Micro-Epsilon are used in the glass industry for different High accuracy measurement tasks: robust eddy current Robust and suitable for industrial applications sensors are integrated into machines in order to detect machine movements while optical sensors monitor glass products in processing lines. Typical measured para- meters include displacement, position, thermoIMAGER / thermoMETER thickness, color and temperature. Thermal imaging cameras and infrared pyrometers for non-contact temperature measurement Fast and precise temperature measurement Real-time process monitoring and system control Compact design & extensive range of interfaces optoNCDT 1420 Compact laser triangulation displacement sensor for high speed, precision measurements Non-contact displacement and distance measurements with large measuring ranges from 10mm to 500mm High accuracy High measuring rate for dynamic measurements Compact design and easy to install Flat glass Temperature measurement of float glass After the tin bath, flat glass has a temperature of approx.