Supporting Evidence for Alternate Cefepime Dosing Substitution
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Antimicrobial Stewardship Guidance
Antimicrobial Stewardship Guidance Federal Bureau of Prisons Clinical Practice Guidelines March 2013 Clinical guidelines are made available to the public for informational purposes only. The Federal Bureau of Prisons (BOP) does not warrant these guidelines for any other purpose, and assumes no responsibility for any injury or damage resulting from the reliance thereof. Proper medical practice necessitates that all cases are evaluated on an individual basis and that treatment decisions are patient-specific. Consult the BOP Clinical Practice Guidelines Web page to determine the date of the most recent update to this document: http://www.bop.gov/news/medresources.jsp Federal Bureau of Prisons Antimicrobial Stewardship Guidance Clinical Practice Guidelines March 2013 Table of Contents 1. Purpose ............................................................................................................................................. 3 2. Introduction ...................................................................................................................................... 3 3. Antimicrobial Stewardship in the BOP............................................................................................ 4 4. General Guidance for Diagnosis and Identifying Infection ............................................................. 5 Diagnosis of Specific Infections ........................................................................................................ 6 Upper Respiratory Infections (not otherwise specified) .............................................................................. -

Penicillin Allergy Guidance Document
Penicillin Allergy Guidance Document Key Points Background Careful evaluation of antibiotic allergy and prior tolerance history is essential to providing optimal treatment The true incidence of penicillin hypersensitivity amongst patients in the United States is less than 1% Alterations in antibiotic prescribing due to reported penicillin allergy has been shown to result in higher costs, increased risk of antibiotic resistance, and worse patient outcomes Cross-reactivity between truly penicillin allergic patients and later generation cephalosporins and/or carbapenems is rare Evaluation of Penicillin Allergy Obtain a detailed history of allergic reaction Classify the type and severity of the reaction paying particular attention to any IgE-mediated reactions (e.g., anaphylaxis, hives, angioedema, etc.) (Table 1) Evaluate prior tolerance of beta-lactam antibiotics utilizing patient interview or the electronic medical record Recommendations for Challenging Penicillin Allergic Patients See Figure 1 Follow-Up Document tolerance or intolerance in the patient’s allergy history Consider referring to allergy clinic for skin testing Created July 2017 by Macey Wolfe, PharmD; John Schoen, PharmD, BCPS; Scott Bergman, PharmD, BCPS; Sara May, MD; and Trevor Van Schooneveld, MD, FACP Disclaimer: This resource is intended for non-commercial educational and quality improvement purposes. Outside entities may utilize for these purposes, but must acknowledge the source. The guidance is intended to assist practitioners in managing a clinical situation but is not mandatory. The interprofessional group of authors have made considerable efforts to ensure the information upon which they are based is accurate and up to date. Any treatments have some inherent risk. Recommendations are meant to improve quality of patient care yet should not replace clinical judgment. -
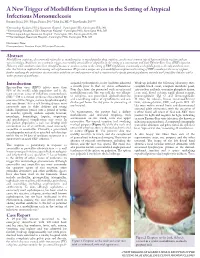
A New Trigger of Morbilliform Eruption in the Setting of Atypical Infectious
A New Trigger of Morbilliform Eruption in the Setting of Atypical Infectious Mononucleosis Roxanne Rajaii, DO,* Megan Furniss, DO,** John Pui, MD,*** Brett Bender, DO**** *Dermatology Resident, PGY2, Beaumont Hospital - Farmington Hills, Farmington Hills, MI **Dermatology Resident, PGY4, Beaumont Hospital - Farmington Hills, Farmington Hills, MI ***Dermatopathologist, Beaumont Hospital - Farmington Hills, Farmington Hills, MI ****Dermatologist, Beaumont Hospital - Farmington Hills, Farmington Hills, MI Disclosures: None Correspondence: Roxanne Rajaii, DO; [email protected] Abstract Morbilliform eruptions, also commonly referred to as exanthematous or maculopapular drug eruptions, are the most common type of hypersensitivity reaction and can vary in etiology. Antibiotics are a common trigger, most notably amoxicillin or ampicillin in the setting of a concomitant and acute Epstein-Barr virus (EBV) infection. However, other antibiotics have been identified to cause a similar reaction in the setting of EBV. Cephalexin, a commonly used cephalosporin, is the only antibiotic in its class that has been implicated in causing such a phenomenon. We present a unique case of a morbilliform eruption in the setting of EBV secondary to the use of cefepime, further outlining the importance of conservative antibiotic use and awareness of such a reaction in this specific patient population, not only with penicillins but also with a wider spectrum of antibiotics. Introduction acquired pyelonephritis, as she had been admitted Work-up included the following laboratory tests: Epstein-Barr virus (EBV) infects more than a month prior to that for status asthmaticus. complete blood count, complete metabolic panel, 98% of the world’s adult population and is the Four days later, she presented with an urticarial anti-nuclear antibody, creatinine phosphate kinase, most common cause of infectious mononucleosis morbilliform rash. -

ESBL Disc Tests - Rev.2 / 19.06.2014 ESBL Disc Tests Disc Tests for Detection of ESBL-Producing Enterobacteriaceae
© Liofilchem® - ESBL Disc Tests - Rev.2 / 19.06.2014 ESBL Disc Tests Disc tests for detection of ESBL-producing Enterobacteriaceae. DESCRIPTION Extended-spectrum β-lactamases (ESBLs) are enzymes hydrolyzing most penicillins and cephalosporins, including oxyimino- β-lactam compunds but not cephamycins and carbapenems. Most ESBLs belong to the Amber class A of β-lactamases and are inhibited by β-lactamases inhibitors: clavulanic acid, sulbatam and tazobactam. ESBL production has been observed mostly in Enterobacteriaceae, particularly Escherichia coli and Klebsiella pnuemoniae, but all other clinically-relevant Enterobacteriaceae species are also common ESBL-producers. In many areas, ESBL detection and characterization is recommended or mandatory for infection control purpose. ESBL detection involves two important steps. The first is a screening test with an indicator cephalosporin which looks for resistance or diminished susceptibility, thus identifying isolates likely to be harboring ESBLs. The second step is a confirmation test which evaluates the synergy between an oxyimino cephalosporin and clavulanic acid, distinguishing isolates with ESBLs from those that are resistant for other reasons. METHOD PRINCIPLE ESBL Disc Screening Disk-diffusion method for ESBL screening can be performed using cefpodoxime, ceftazidime, aztreonam, cefotaxime and ceftriaxone, according to EUCAST and/or CLSI guidelines (table 1). Since the affinity of ESBLs for different substrates is variable, the use of more than one of these agents for screening improves the sensitivity of detection. However, it is adequate to use the couple cefotaxime (or ceftriaxone) and ceftazidime. If only one drug can be used, cefpodoxime is the most sensitive indicator cephalosporin for detection of ESBL production may be used for screening. -

MICU Antibiotics and Associated Drug Interactions
MICU Antibiotics and Associated Drug Interactions Resistant Bacteria ► MICU patient are at risk for resistant organisms: § Recent hospitalizations § From a skilled nursing facility § Immunocompromised patients ►Transplant population ►Chronic steroid use Organisms of Concern ► Gram Negative organisms § Pseudomonas aeruginosa § Acinetobacter § Klebsiella pneumoniae ► ESBL § E.Coli ► ESBL ► Staph Aureus § MRSA ► Enterococcus faecalis & faecium § VRE Antibiotic Management Program (AMP) ► Patient safety initiative to address: § The infections related to C. difficile § Increasing antimicrobial resistance § Increasing antimicrobial cost Commonly Used Restricted Antibiotics ► Must call AMP to get an approval code (Full list on page 12 of Guide to Antimicrobial Chemotherapy) § Commonly used agents ► Ciprofloxacin ► Moxifloxacin ► Linezolid ► Ceftriaxone ► Ceftazidime ► Aztreonam ► Daptomycin ► Clindamycin ► Meropenem ► Imipenem ► Oral Vancomycin—unless you fill out a C.diff order set Not Restricted in the ICU ► Vancomycin ► Zosyn (piperacilin/tazobactam) ► Cefepime ► Aminoglycosides § Gentamicin § Tobramycin Pharmacodynamics ► Concentration dependant killing ► Goal: maximize the concentration § AUC/MIC ratio and Peak/MIC are predictors of efficacy § Antibiotics kill with increasing antibiotic concentrations at a greater rate and extent § Aminoglycosides ►Fluoroquinolones ►Metronidazole Pharmacodynamics ► Time dependent killing ► Goal: Maximize duration of exposure § T > MIC correlates best with efficacy ►Antibiotics kill bacteria at the same rate -

Pharmacy Update: Truth and Consequences of Beta-Lactam Allergy Management
Pharmacy Update: Truth and Consequences of Beta-Lactam Allergy Management Penicillin (PCN) Allergy Background o PCN allergy is the most common drug-class allergy reported - ~8-12% of patients self-report a PCN allergy - Reported anaphylactic reaction to PCN commonly precludes prescribers from using β-lactams in these patients o 80-90% of patients reporting a PCN allergy will have a negative response to PCN skin testing Impact of Penicillin Allergies o Penicillin “allergies” lead to: - More costly, less effective therapy o Longer length of stay, more medications used, more treatment failures - Worse clinical outcomes o Increased mortality, more treatment failures - 2nd and 3rd line antibiotics commonly substituted for β-lactams in patients with a penicillin “allergy” o Suboptimal use of fluoroquinolones, clindamycin, vancomycin, and aztreonam (e.g. vancomycin for MSSA) o Macy et al. J Allergy Clin Immunol: - Significantly more fluoroquinolone, clindamycin, and vancomycin use - 23.4% more C. difficile (95% CI: 15.6%-31.7%) - 14.1% more MRSA (95% CI: 7.1%-21.6%) - 30.1% more VRE infections (95% CI: 12.5%-50.4%) The Myth of Cross-Reactivity between Penicillins & Cephalosporins o The widely quoted cross-reactivity rate of 10% was originally reported in the 1960s, studies were flawed due to cephalosporins being frequently contaminated with penicillin o More recent observational studies have Table 1. FDA-approved Beta-lactam Antibiotics with Similar Side Chainsa found cross-reactivity rates between 0.17% Agent Agents with Similar Side Chains -

Off-Label Use of Ceftaroline Fosamil: a Systematic Review
International Journal of Antimicrobial Agents 54 (2019) 562–571 Contents lists available at ScienceDirect International Journal of Antimicrobial Agents journal homepage: www.elsevier.com/locate/ijantimicag Review Off-label use of ceftaroline fosamil: A systematic review ∗ Arianna Pani a,e, , Fabrizio Colombo b, Francesca Agnelli b, Viviana Frantellizzi c, Francesco Baratta d, Daniele Pastori d, Francesco Scaglione a,e a Clinical Pharmacology Unit, ASST Grande Ospedale Metropolitano Niguarda, Italy b Internal Medicine Department, ASST Grande Ospedale Metropolitano Niguarda, Italy c Department of Radiological, Oncological and Anatomical Pathological Sciences, University of Rome Sapienza, Italy d Department of Internal Medicine and Medical Specialties, University of Rome Sapienza, Italy e Department of Oncology and Onco-Hematology, Postgraduate School of Clinical Pharmacology and Toxicology University of Milan Statale, Italy a r t i c l e i n f o a b s t r a c t Article history: Ceftaroline fosamil is a fifth-generation cephalosporin with anti-methicillin-resistant Staphylococcus au- Received 14 December 2018 reus (MRSA) activity. It has been approved by the EMA and FDA for the treatment of adults and children Accepted 28 June 2019 with community-acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure in- fections (ABSSSI). However, ceftaroline fosamil has a broad spectrum of activity, and a good safety and Editor: Stefania Stefani tolerability profile, so is frequently used off-label. The aim of this systematic review was to summarize the safety and efficacy of off-label use of cef- Keywords: Ceftaroline fosamil taroline. MRSA The review was conducted according to PRISMA guidelines. MEDLINE, EMBASE and CENTRAL Bacteremia databases (2010-2018) were searched using as the main term ceftaroline fosamil and its synonyms in Endocarditis combination with names of infectious diseases of interest. -

Guidelines for Treatment of Intra-Abdominal Infections in Adults
GUIDELINES FOR TREATMENT OF INTRA-ABDOMINAL INFECTIONS IN ADULTS Table of Contents Appendicitis Cholangitis & Cholecystitis Diverticulitis Esophageal Perforation Secondary Peritonitis Tertiary Peritonitis (Infection associated with perforation or spillage of GI pathogens into (Persistent infection associated with recurring GI perforation and/or the peritoneal cavity) anastomotic leakage after initial treatment for secondary peritonitis) Spontaneous Bacterial Peritonitis (SBP) Treatment and Prophylaxis Pancreatitis Footnotes References Table of Contents Appendicitis Empiric Therapy Duration Community Acquired, No Severe Sepsis/Shock Non-perforated: 1st line: Discontinue after appendectomy. If no appendectomy performed a 10-day duration is Cefuroxime* 1.5 g IV q8h recommended ref1 + Metronidazole 500 mg PO/IV q8h High-risk allergy3/contraindications4 to beta-lactams: Perforated: Ciprofloxacin* 400 mg IV q8h 4 full days after source control ref 3 + Metronidazole 500 mg PO/IV q8h Duration of therapy may be extended with inadequate source control or persistent Community Acquired with Severe Sepsis/Shock OR MDR-GNR Risk: clinical symptoms or signs of infection. st 1 line: Piperacillin-tazobactam*4.5 g IV q6h Patients with bacteremia: 2 Low/medium-risk allergy to penicillins: 7-14 days Cefepime* 2 g IV q8h + Metronidazole 500 mg PO/IV q8h For patients with secondary gram-negative bacteremia, a 7-day duration of IV therapy Consider the addition of vancomycin to cefepime for Enterococcus (or oral quinolone at discharge) may be appropriate ref5 -

Antibiotic Shortages in Pediatrics MEDICATION SHORTAGES ARE INCREASING NATIONWIDE
Ritu Banerjee, MD, PhD, a Cary W. Thurm, PhD, b Erin R. Fox, PharmD, c Adam L. Hersh, MD, PhDc Antibiotic Shortages in Pediatrics MEDICATION SHORTAGES ARE INCREASING NATIONWIDE Medication shortages are increasingly common and severe in the United States and are a serious health threat. Medication shortages occur when reduced supply of a drug influences how1 it is prepared, dispensed, or prescribed by pharmacies or providers. According to the US Food and Drug Administration (FDA), most shortages are caused by quality and 2 aVanderbilt University Medical Center, Nashville, Tennessee; manufacturing problems. Consequences of shortages include rationing bChildren’s Hospital Association, Lenexa, Kansas; and cUniversity of Utah, Salt Lake City, Utah limited supplies, delays in therapy,3 medication errors, use of less- efficacious alternatives, and death. Recently, critical drugs like sodium Drs Banerjee and Hersh conceptualized and designed chloride and amino acids have been on shortage, exacerbated by damage the study, drafted the initial manuscript, and reviewed to manufacturing plants by natural disasters like Hurricane Maria. and revised the manuscript; Dr Thurm collected the data, Although shortages have affected virtually all classes of medications, performed the initial analyses, and reviewed and revised the antimicrobial shortages are common and account for 15% of all drug manuscript; Dr Fox critically reviewed the manuscript for 1, 4 important intellectual content and revised the manuscript; shortages. and all authors approved the final manuscript as submitted ANTIMICROBIAL SHORTAGES CAN CAUSE HARM TO PATIENTS, AND THEIR and agree to be accountable for all aspects of the work. IMPACT ON CHILDREN IS NOT WELL STUDIED DOI: https:// doi. -

“CRE Mechanisms and Their Importance for Infection Prevention ” Paul C
MICHIGAN DEPARTMENT OF COMMUNITY HEALTH June 25, 2015 “CRE Mechanisms and their Importance for Infection Prevention ” Paul C. Schreckenberger, Ph.D., D(ABMM), F(AAM) Professor of Pathology Director, Clinical Microbiology Laboratory Loyola University Medical Center [email protected] Financial Disclosures Type of Financial Interest Name of Commercial Interest Salaried Employee Loyola University Medical Center Stocks/Stock Options None Independent Accelerate Dx., Beckman Coulter, contractor/Speaker’s bioMerieux, BioFire, Cepheid, Hardy Bureau Diagnostics, Merck, Thermo Fisher Scientific Consultant/Advisory BioFire, Cempra, Cepheid, GenMark, Committees Quidel, Thermo Fisher Scientific, Theravance Research Grants Accelerate Dx, Becton-Dickinson, Beckman Coulter, BioFire, bioMerieux, Bruker, Cepheid, Learning Objectives At the conclusion of this session, participants will be able to: 1.Describe the five major types of CRE 2.Review conventional and new approaches to detecting CRE 3.Explain the CSTE CRE definition proposal and its implications for labs 4.Evaluate their own laboratories readiness for detecting and reporting CRE 3 Penicillin nucleus S R 1 CH3 5 2 6 CH3 7 4 N 3 O COOH Cephalosporin nucleus 1 S 7 R1 C HN R O O 2 COOH4 13 MODE OF ACTION OF BETA LACTAMS IN GRAM NEGATIVES SUSCEPTIBLE RESISTANT - Lactam Antibiotic Diffusion through Porin Blocks Entry Outer Membrane Efflux Pump Diffusion through Beta-Lactamase Peptidoglycan Hydolyzes Beta-Lactam Penicillin Binding Proteins Changes in PBP results in Cell Death Failure to Bind -

Cefepime and Ceftazidime Safety in Hospitalized Infants Cefepime and Ceftazidime Safety Christopher J
Jayachitra ANTIMICROBIAL REPORTS PIDJ PIDJ-214-789 Cefepime and Ceftazidime Safety in Hospitalized Infants Cefepime and Ceftazidime Safety Christopher J. Arnold, MD,*† Jessica Ericson, MD,*‡ Nathan Cho,* James Tian,* Shelby Wilson,* Vivian H. Chu, MD, MHS,*† Christoph P. Hornik, MD, MPH,*‡ Reese H. Clark, MD,§ Arnold et al Daniel K. Benjamin Jr, MD, PhD, MPH,*‡ and P. Brian Smith, MD, MPH, MHS,*‡ on behalf of the Best Pharmaceuticals for Children Act – Pediatric Trials Network Administrative Core Committee XXX between the 2 groups. There was no difference in seizure risk or mortality Background: Cefepime and ceftazidime are cephalosporins used for the between the 2 drugs. Pediatr Infect Dis J treatment of serious Gram-negative infections. These cephalosporins are used off-label in the setting of minimal safety data for young infants. (Pediatr Infect Dis J 2015;34:964–968) Lippincott Williams & Wilkins Methods: We identified all infants discharged from 348 neonatal intensive care units managed by the Pediatrix Medical Group between 1997 and 2012 Hagerstown, MD who were exposed to either cefepime or ceftazidime in the first 120 days of life. We reported clinical and laboratory adverse events occurring in infants ephalosporins are one of the most widely used classes of anti- exposed to cefepime or ceftazidime and used multivariable logistic regres- Cbiotics. Their broad spectrum, which includes both Gram- sion to compare the odds of seizures and death between the 2 groups. positive and Gram-negative organisms, coupled with an overall low Results: A total of 1761 infants received 13,293 days of ceftazidime, and toxicity profile have made cephalosporins a popular choice for both 1 594 infants received 4628 days of cefepime. -
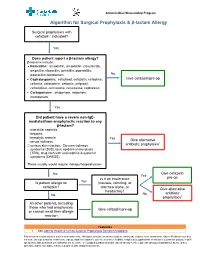
Algorithm for Surgical Prophylaxis & Β-Lactam Allergy
Antimicrobial Stewardship Program Algorithm for Surgical Prophylaxis & β-lactam Allergy Surgical prophylaxis with cefazolin1 indicated? Yes Does patient report a β-lactam allergy? β-lactams include: • Penicillins: amoxicillin, amoxicillin- clavulanate, ampicillin, cloxacillin, penicillin, piperacillin, piperacillin-tazobactam No • Cephalosporins: cefadroxil, cefazolin, cefepime, Give cefazolin pre-op cefixime, cefotaxime, cefoxitin, cefprozil, ceftazidime, ceftriaxone, cefuroxime, cephalexin • Carbapenems: ertapenem, imipenem, meropenem Yes Did patient have a severe non-IgE- mediated/non-anaphylactic reaction to any β-lactam? interstitial nephritis hepatitis hemolytic anemia Yes Give alternative serum sickness 1 serious skin reaction: Stevens-Johnson antibiotic prophylaxis syndrome (SJS), toxic epidermal necrolysis (TEN), drug rash with eosinophilia & systemic symptoms (DRESS) These usually would require therapy/hospitalization. No Give cefazolin Yes Is it an intolerance pre-op Is patient allergic to Yes (nausea, vomiting, or cefazolin? diarrhea alone, or Give alternative headache)? antibiotic No No prophylaxis1 All other patients, including those who had anaphylaxis Give cefazolin pre-op or cannot recall their allergic reaction Footnotes 1. See Alberta Health Services Surgical Prophylaxis Recommendations This information is intended for general information only. Although reasonable efforts were made to confirm the accuracy of the information, Alberta Health Services does not make any representation or warranty, express, implied or statutory, as to the accuracy, reliability, completeness, applicability or fitness for a particular purpose of such information. This material is not a substitute for the advice of a qualified health professional. Alberta Health Services expressly disclaims all liability for the use of these materials, and for any claims, actions, demands or suits arising from such use. Antimicrobial Stewardship Program Q&A: Surgical Prophylaxis & β-lactam Allergy See β-lactam Allergy Antimicrobial Stewardship Backgrounder Q1.