Some Generic Changes in Arctiinae from South Eurasia with The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![Artimelia Latreillei (Godart, [1823] 1822) VU](https://docslib.b-cdn.net/cover/4404/artimelia-latreillei-godart-1823-1822-vu-214404.webp)
Artimelia Latreillei (Godart, [1823] 1822) VU
Libro Rojo de los Invertebrados de España Artimelia latreillei (Godart, [1823] 1822) VU Categoría UICN: Vulnerable Criterio UICN: B1b(ii,iii,iv,v)c(ii,iii,iv) Nombre Vulgar: Tipo: Arthropoda Clase: Insecta Orden: Lepidoptera Familia: Arctiidae Área de distribución Elemento faunístico atlanto-mediterráneo, endé- ocre-marrón junto con dos prominencias amarillas mico de la península Ibérica (España y Portugal). a cada lado del primer segmento torácico. Las patas Sus colonias, aunque repartidas por buena parte son ocre-anaranjado-pálidas. El cuerpo es cilíndrico de la geográfica española y portuguesa, son siem- con una nítida línea dorsal blanca, dos finas líneas pre localizadas y dispersas. En concreto en España latero-dorsales azul claro mate y dos líneas laterales ha sido repetidamente citada de Cataluña (Sega- ocre-anaranjadas con verrugas grises. La piel es ne- rra, 1997), Aragón (Redondo, 1990; Murria, 1995) gra, con verrugas también negras simétricamente y Galicia (Fernández-Vidal et al., 1992). Existen repartidas en cada segmento, de las cuales salen además citas aisladas para Castellón (Calle, 1983), abundantes sedas negras y blanquecinas. En el últi- Huelva (Huertas, 1984), León (Vega, 1980), Valla- mo estadío puede llegar hasta los 30 mm de longi- dolid (Magro, 1989), Álava (Olano et al., 1986), tud. Son muy activas y polífagas, alimentándose du- Cuenca (Aistleitner y Aistleitner, 1998) y Cáceres rante mayo y junio, tanto de las hojas como de los (Blázquez et al., 1997), así como para la Sierra de pétalos, de especies pertenecientes a los géneros Guadarrama y Sierra Morena (Gómez de Aizpú- Plantago, Genista, Sonchus, Picridium, Spartium, Sca- rua, 1974; García de Viedma y Gómez Bustillo, biosa, Cytisus, Taraxacum, Senecio y Rumex. -

Redalyc.New and Interesting Portuguese Lepidoptera Records from 2007 (Insecta: Lepidoptera)
SHILAP Revista de Lepidopterología ISSN: 0300-5267 [email protected] Sociedad Hispano-Luso-Americana de Lepidopterología España Corley, M. F. V.; Marabuto, E.; Maravalhas, E.; Pires, P.; Cardoso, J. P. New and interesting Portuguese Lepidoptera records from 2007 (Insecta: Lepidoptera) SHILAP Revista de Lepidopterología, vol. 36, núm. 143, septiembre, 2008, pp. 283-300 Sociedad Hispano-Luso-Americana de Lepidopterología Madrid, España Available in: http://www.redalyc.org/articulo.oa?id=45512164002 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative 283-300 New and interesting Po 4/9/08 17:37 Página 283 SHILAP Revta. lepid., 36 (143), septiembre 2008: 283-300 CODEN: SRLPEF ISSN:0300-5267 New and interesting Portuguese Lepidoptera records from 2007 (Insecta: Lepidoptera) M. F. V. Corley, E. Marabuto, E. Maravalhas, P. Pires & J. P. Cardoso Abstract 38 species are added to the Portuguese Lepidoptera fauna and two species deleted, mainly as a result of fieldwork undertaken by the authors in the last year. In addition, second and third records for the country and new food-plant data for a number of species are included. A summary of papers published in 2007 affecting the Portuguese fauna is included. KEY WORDS: Insecta, Lepidoptera, geographical distribution, Portugal. Novos e interessantes registos portugueses de Lepidoptera em 2007 (Insecta: Lepidoptera) Resumo Como resultado do trabalho de campo desenvolvido pelos autores principalmente no ano de 2007, são adicionadas 38 espécies de Lepidoptera para a fauna de Portugal e duas são retiradas. -

Recerca I Territori V12 B (002)(1).Pdf
Butterfly and moths in l’Empordà and their response to global change Recerca i territori Volume 12 NUMBER 12 / SEPTEMBER 2020 Edition Graphic design Càtedra d’Ecosistemes Litorals Mediterranis Mostra Comunicació Parc Natural del Montgrí, les Illes Medes i el Baix Ter Museu de la Mediterrània Printing Gràfiques Agustí Coordinadors of the volume Constantí Stefanescu, Tristan Lafranchis ISSN: 2013-5939 Dipòsit legal: GI 896-2020 “Recerca i Territori” Collection Coordinator Printed on recycled paper Cyclus print Xavier Quintana With the support of: Summary Foreword ......................................................................................................................................................................................................... 7 Xavier Quintana Butterflies of the Montgrí-Baix Ter region ................................................................................................................. 11 Tristan Lafranchis Moths of the Montgrí-Baix Ter region ............................................................................................................................31 Tristan Lafranchis The dispersion of Lepidoptera in the Montgrí-Baix Ter region ...........................................................51 Tristan Lafranchis Three decades of butterfly monitoring at El Cortalet ...................................................................................69 (Aiguamolls de l’Empordà Natural Park) Constantí Stefanescu Effects of abandonment and restoration in Mediterranean meadows .......................................87 -
![Ichneumonid Wasps (Hymenoptera, Ichneumonidae) in the to Scale Caterpillar (Lepidoptera) [1]](https://docslib.b-cdn.net/cover/0863/ichneumonid-wasps-hymenoptera-ichneumonidae-in-the-to-scale-caterpillar-lepidoptera-1-720863.webp)
Ichneumonid Wasps (Hymenoptera, Ichneumonidae) in the to Scale Caterpillar (Lepidoptera) [1]
Central JSM Anatomy & Physiology Bringing Excellence in Open Access Research Article *Corresponding author Bui Tuan Viet, Institute of Ecology an Biological Resources, Vietnam Acedemy of Science and Ichneumonid Wasps Technology, 18 Hoang Quoc Viet, Cau Giay, Hanoi, Vietnam, Email: (Hymenoptera, Ichneumonidae) Submitted: 11 November 2016 Accepted: 21 February 2017 Published: 23 February 2017 Parasitizee a Pupae of the Rice Copyright © 2017 Viet Insect Pests (Lepidoptera) in OPEN ACCESS Keywords the Hanoi Area • Hymenoptera • Ichneumonidae Bui Tuan Viet* • Lepidoptera Vietnam Academy of Science and Technology, Vietnam Abstract During the years 1980-1989,The surveys of pupa of the rice insect pests (Lepidoptera) in the rice field crops from the Hanoi area identified showed that 12 species of the rice insect pests, which were separated into three different groups: I- Group (Stem bore) including Scirpophaga incertulas, Chilo suppressalis, Sesamia inferens; II-Group (Leaf-folder) including Parnara guttata, Parnara mathias, Cnaphalocrocis medinalis, Brachmia sp, Naranga aenescens; III-Group (Bite ears) including Mythimna separata, Mythimna loryei, Mythimna venalba, Spodoptera litura . From these organisms, which 15 of parasitoid species were found, those species belonging to 5 families in of the order Hymenoptera (Ichneumonidae, Chalcididae, Eulophidae, Elasmidae, Pteromalidae). Nine of these, in which there were 9 of were ichneumonid wasp species: Xanthopimpla flavolineata, Goryphus basilaris, Xanthopimpla punctata, Itoplectis naranyae, Coccygomimus nipponicus, Coccygomimus aethiops, Phaeogenes sp., Atanyjoppa akonis, Triptognatus sp. We discuss the general biology, habitat preferences, and host association of the knowledge of three of these parasitoids, (Xanthopimpla flavolineata, Phaeogenes sp., and Goryphus basilaris). Including general biology, habitat preferences and host association were indicated and discussed. -

ÇUKUROVA ÜNİVERSİTESİ FEN BİLİMLERİ ENSTİTÜSÜ Papatya
ÇUKUROVA ÜNİVERSİTESİ FEN BİLİMLERİ ENSTİTÜSÜ YÜKSEK LİSANS TEZİ Papatya DEMİREZER BALCALI (ADANA)’DA FARKLI HABİTATLARDAKİ GECE AKTİF LEPİDOPTERA TÜRLERİ VE BİYOLOJİK ÇEŞİTLİLİĞİ ÜZERİNDE ARAŞTIRMALAR BİTKİ KORUMA ANABİLİM DALI ADANA, 2006 ÇUKUROVA ÜNİVERSİTESİ FEN BİLİMLERİ ENSTİTÜSÜ BALCALI (ADANA)’DA FARKLI HABİTATLARDAKİ GECE AKTİF LEPİDOPTERA TÜRLERİ VE BİYOLOJİK ÇEŞİTLİLİĞİ ÜZERİNDE ARAŞTIRMALAR Papatya DEMİREZER YÜKSEK LİSANS TEZİ BİTKİ KORUMA ANABİLİM DALI Bu Tez 24/11/2006 Tarihinde Aşağıdaki Jüri Üyeleri Tarafından Oybirliği İle Kabul Edilmiştir. İmza:................................ İmza:.............................. İmza:................................... Prof.Dr. Serpil KORNOŞOR Prof.Dr. M. Rifat ULUSOY Yard.Doç.Dr. Erdal SERTKAYA DANIŞMAN ÜYE ÜYE Bu Tez Enstitümüz Bitki Koruma Anabilim Dalında Hazırlanmıştır. Kod No: Prof. Dr. Aziz ERTUNÇ Enstitü Müdürü Bu Çalışma Çukurova Üniversitesi Bilimsel Araştırma Projeleri Birimi Tarafından Desteklenmiştir. Proje No: ZF2004YL74 Not: Bu tezde kulanılan özgün ve başka kaynaktan yapılan bildirişlerin, çizelge, şekil ve fotoğrafların kaynak gösterilmeden kullanımı, 5846 sayılı Fikir ve Sanat Eserleri Kanunundaki hükümlere tabidir. ÖZ YÜKSEK LİSANS TEZİ BALCALI (ADANA)’DA FARKLI HABİTATLARDAKİ GECE AKTİF LEPİDOPTERA TÜRLERİ VE BİYOLOJİK ÇEŞİTLİLİĞİ ÜZERİNDE ARAŞTIRMALAR Papatya DEMİREZER ÇUKUROVA ÜNİVERSİTESİ FEN BİLİMLERİ ENSTİTÜSÜ BİTKİ KORUMA ANABİLİM DALI Danışman: Prof. Dr. Serpil KORNOŞOR Yıl: 2006, Sayfa:70 Jüri: Prof. Dr. Serpil KORNOŞOR : Prof. Dr. M. Rifat ULUSOY : Yard. -

“Phragmatobia” (Erebidae, Arctiinae)
A peer-reviewed open-access journal ZooKeys 149:Generic 69–88 placement (2011) of the Neotropical species of “Phragmatobia” (Erebidae, Arctiinae)... 69 doi: 10.3897/zookeys.149.2382 RESEARCH ARTICLE www.zookeys.org Launched to accelerate biodiversity research Generic placement of the Neotropical species of “Phragmatobia” (Erebidae, Arctiinae), with a remarkable matrivorous species from the Peruvian Andes B. Christian Schmidt1,†, Josef J. De Freina2,‡ 1 Canadian Food Inspection Agency, Canadian National Collection of Insects, Arachnids and Nematodes, K.W. Neatby Bldg., 960 Carling Ave., Ottawa, ON, Canada K1A 0C6 2 Eduard-Schmid Str. 10, D-81541, Munich, Germany † urn:lsid:zoobank.org:author:C3C5392A-EBF8-41B9-99BE-364A8C2FBB7F ‡ urn:lsid:zoobank.org:author:D77A3D39-F4A4-4116-8279-5F6534826BE8 Corresponding authors: B. Christian Schmidt ([email protected]), Josef J. De Freina ([email protected]) Academic editor: D. Lafontaine | Received 10 September 2011 | Accepted 15 November 2011 | Published 24 November 2011 urn:lsid:zoobank.org:pub:6476A2E7-14C0-4E6E-B21E-4B63375BF605 Citation: Schmidt BC, De Freina JJ (2011) Generic placement of the Neotropical species of “Phragmatobia” (Erebidae, Arctiinae), with a remarkable matrivorous species from the Peruvian Andes. In: Schmidt BC, Lafontaine JD (Eds) Contributions to the systematics of New World macro-moths III. ZooKeys 149: 69–88. doi: 10.3897/zookeys.149.2382 Abstract Phragmatobia Stephens is briefly reviewed and a diagnosis is provided. The South American species cur- rently placed in Phragmatobia Stephens are revised to two new genera, Andesobia Schmidt and De Freina, gen. n., and Patagobia Schmidt and De Freina, gen. n. (subtribe Spilosomina). Both Andesobia and Patagobia exhibit adaptations to high altitude habitats, including micropterous females in Andesobia (Patagobia females are unknown) and diurnal flight of males. -

Federal Register July 23,1996 Tuesday 38137 II Federal Register / Vol
7±23±96 Tuesday Vol. 61 No. 142 July 23, 1996 Pages 38049±38352 federal register 38137 II Federal Register / Vol. 61, No. 142 / Tuesday, July 23, 1996 SUBSCRIPTIONS AND COPIES PUBLIC Subscriptions: Paper or fiche 202±512±1800 FEDERAL REGISTER Published daily, Monday through Friday, Assistance with public subscriptions 512±1806 (not published on Saturdays, Sundays, or on official holidays), by General online information 202±512±1530 the Office of the Federal Register, National Archives and Records Administration, Washington, DC 20408, under the Federal Register Single copies/back copies: Act (49 Stat. 500, as amended; 44 U.S.C. Ch. 15) and the Paper or fiche 512±1800 regulations of the Administrative Committee of the Federal Register Assistance with public single copies 512±1803 (1 CFR Ch. I). Distribution is made only by the Superintendent of Documents, U.S. Government Printing Office, Washington, DC FEDERAL AGENCIES 20402. Subscriptions: The Federal Register provides a uniform system for making Paper or fiche 523±5243 available to the public regulations and legal notices issued by Assistance with Federal agency subscriptions 523±5243 Federal agencies. These include Presidential proclamations and For other telephone numbers, see the Reader Aids section Executive Orders and Federal agency documents having general applicability and legal effect, documents required to be published at the end of this issue. by act of Congress and other Federal agency documents of public interest. Documents are on file for public inspection in the Office of the Federal Register the day before they are published, unless earlier filing is requested by the issuing agency. -

Tourbières Des Grandes Rousses – AVENIR - 2005 Annexe D.VI : Tourbières Du Ruisseau Du Bessey
TOURBIERES DU DISTRICT NATUREL DES GRANDES ROUSSES ATLAS DU DOSSIER DE PRISE EN POLITIQUE ESPACES CONSIDERATION NATURELS SENSIBLES DU CONSEIL GENERAL DE L'ISERE NOVEMBRE 2005 AVENIR - Conservatoire départemental des espaces naturels de l’Isère 10 rue Raspail 38000 GRENOBLE Tél. 04 76 48 24 49 Fax 04 76 48 24 26 [email protected] Réalisation : AVENIR Conservatoire des espaces naturels de l'Isère Rédaction : Myrtille Bérenger Céline Balmain Cartographie : Laurent Poulin Coordination : Roger Marciau ATLAS Dossier de prise en considération pour la préservation des tourbières du district des Grandes Rousses (Massif et Emparis) Financement : SOMMAIRE DE L’ATLAS Annexe C.I : Localisation des tourbières dans le district Annexe C.II : Commune de Besse-en-oisans Annexe C.III : Commune de Clavans-en-Oisans Annexe C.IV : Commune du Freney-d’Oisans Annexe C.V : Commune de La Garde-en-Oisans Annexe C.VI : Commune d’Huez Annexe C.VII : Commune de Mizoën Annexe C.VIII : Commune d’Oz-en-Oisans Annexe C.IX : Commune de Vaujany Annexe D.I : Marais du Rif Tort Annexe D.II : Tourbière de la Petite Lauze Annexe D.III : Tourbière de Chavannus Annexe D. IV : Tourbière de la Rochette Annexe D. V : Tourbière de Mont Frais ATLAS - Dossier de prise en considération pour la préservation des tourbières des Grandes Rousses – AVENIR - 2005 Annexe D.VI : Tourbières du ruisseau du Bessey Annexe D.VII : Tourbière de la Pisse Annexe D.VIII : Tourbières du lac Carrelet Annexe D.IX : Tourbières de la Faucille Annexe D.X : Tourbières de la vallée du Ferrand Annexe D.XI : -

Tiger-Moths of Iran 481-525 Atalanta (Dezember 2005) 36 (3/4): 481-525, Würzburg, ISSN 0171-0079
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Atalanta Jahr/Year: 2005 Band/Volume: 36 Autor(en)/Author(s): Dubatolov Vladimir V., Zahiri Reza Artikel/Article: Tiger-moths of Iran 481-525 Atalanta (Dezember 2005) 36 (3/4): 481-525, Würzburg, ISSN 0171-0079 Tiger-moths o f Iran (Lepidoptera, Arctiidae: Arctiinae) by V l a d im ir V. D u b a t o l o v & R e z a Z a h ir i received 26.X.2005 Abstract: Based on the vast material from the collection of the Hayk Mirzayans Insect Museum (HMIM) and literature data, 28 species are recorded from Iran. Callimorpha dominula rossica K o l ., Axiopoena kareliniMtu., Utetheisa lotrixCr ., Watsonarctia deserta B a r t ., Diaphora mendica C l . are recorded from this country for the first time. Four new subspecies, Arctia caja mazandarana subspec. nov. from the Caspian Coast, Eucharia festiva hormozgana subspec. nov. from South Iran, Watsonarctia deserta elbursica subspec. nov. from the Alburz Mts., and Pbragmatobia placida mirzayansi subspec. nov. with a pale coloration, from the high mountains of the Albourz are described. The analysis of the Arctiinae fauna shows that the fauna of South-Eastern Iran is the Oriental, and not Palearctic. Zusammenfassung: Mit Hilfe des reichhaltigen Materials des Hayk Mirzayans Insect Museum (HMIM) und aufgrund von Literaturangaben können 28 Arten für den Iran angegeben werden. Callimorpha dominula rossica K o l ., Axiopoena kareliniM £ n ., Utetheisa lotrix C r ., Watsonarctia deserta B a r t ., Diaphora mendica C l . werden erstmals für dieses Land gemeldet. -
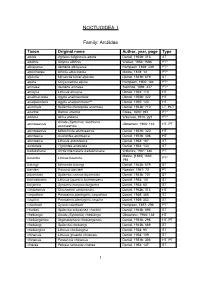
Noctuoidea I
NOCTUOIDEA I Family: Arctiidae Taxon Original name Author, year, page Type albida Agrisius fuliginosus albida Daniel, 1952b: 316 ST albifinis Sidyma albifinis Walker, 1856: 1686 PT* albisparsa Aemene albisparsa Hampson, 1898: 439 PT* albocinerea Ghoria albocinerea Moore, 1878: 13 PT* alpicola Micrarctia hönei alpicola Daniel, 1943b: 679 ST alpina Chrysorabdia alpina Hampson, 1900: 184 PT* amnaea Aemene amnaea Swinhoe, 1894: 437 PT* amoyca Lithosia amoyca Daniel, 1954: 110 HT analimaculata Agylla analimaculata Daniel, 1952b: 322 HT analipunctaria Agylla analipunctaria*** Daniel, 1955: 143 HT anormala Spilarctia rhodophila anormala Daniel, 1943b: 710 LT, PLT ariadne Bizone ariadne Elwes, 1890: 394 PT* arizana Ilema arizana Wileman, 1910: 221 PT* Amata (Syntomis) xanthoma atuntseensis Obraztsov, 1966: 146 HT, PT atuntseensis atuntseensis Miltochrista atuntseensis Daniel, 1951b: 320 HT atuntseica Asurioides atuntseica Daniel, 1951b: 308 HT atuntseica Lithosia antuntseica Daniel, 1954: 107 ST aureolata Tigrioides aureolata Daniel, 1954: 133 ST badakshana Arctia intercalaris badakhshana Wiltshire, 1961: 340 PT Moore, [1866] 1865: basinota Lihosia basinota PT* 798 batangi Micrarctia batangi Daniel, 1943b: 679 ST benderi Paraona benderi Roesler, 1967: 72 PT bipunctata Spilarctia comma bipunctata Daniel, 1943b: 701 ST brunnescens Lithosia japonica brunnescens Daniel, 1954: 101 ST bulgarica Syntomis marjana bulgarica Daniel, 1934: 60 ST cantonensis Chionaema cantonensis Daniel, 1952b: 313 HT carpathica Parasemia plantaginis carpathica Daniel, 1939: -

Insecta) of the Razelm-Sinoe Lagoon Complex (Dobrogea, Romania
J. Wetlands Biodiversity (2018) 8: 113-148 THE MACROLEPIDOPTERA (INSECTA) OF THE RAZELM-SINOE LAGOON COMPLEX (DOBROGEA, ROMANIA) Levente Székely Received: 04.06.2018 / Accepted: 04.07.2018 Abstract: The Danube Delta is one of the most important biosphere reserves of Europe. The most important component of this reserve stands for the Razelm-Sinoe Lagoon Complex placed in the southern part of the Danube Delta. In this work are shown the outmost results of Macrolepidoptera fauna (Insecta: Lepidoptera) of this region, based on faunistic research carried out during 2007-2018, in over 40 collecting trips. 552 species of Macrolepidoptera are listed, and as a new approach, the species are presented according to the most characteristic habitats they can be found in. The most important Macrolepidoptera species in this area are Megaspilates mundataria (Stoll, 1782), Eublemma porphyrinia (Freyer, 1845) and Cucullia argentina (Fabricius, 1787), only to be found in Europe in this region (on The European Union territory). A special attention shall be paid to a series of very rare species, located in this region, that are known in Romania, especially on the territory of the Razelm-Sinoe Lagoon Complex, e.g.: Lasiocampa eversmanni (Eversmann, 1843), Narraga tessularia (Metzner, 1845), Chariaspilates formosaria (Eversmann, 1837), Scopula orientalis (Alphéraky, 1876), Idaea sericeata (Hubner, [1813]), Eupithecia variostrigata (Alphéraky, 1876), Eupithecia biornata (Christoph, 1867), Ocnogyna parasita (Hübner, 1790), Rhyparioides metelkana (Lederer, 1861), Grammodes bifasciata (Petagna, 1787), Clytie syriaca (Bugnion, 1837), Leucania punctosa (Treitscke, 1825), Protarchanara brevilinea (Fenn, 1864), Dychagyris melanura (Kollar, 1846), Cervina (Gortyna) cervago (Eversmann, 1844), Cucullia biornata (Fischer v. Waldheim, 1840), Episema lederi (Christoph, 1885), Saragossa siccanorum (Staudinger, 1870), Saragossa porosa (Eversmann, 1854), Cardepia hartigi (Parenzan, 1981), Agrotis desertorum (Boisduval, 1840) etc. -

Artificial Lighting and Biodiversity in Switzerland
Artificial lighting and Biodiversity in Switzerland Technical Report V4 - Jan 2019 James Hale and Raphaël Arlettaz University of Bern 1 Table of Contents 1 Introduction .....................................................................................................................4 1.1 Background to this report .........................................................................................4 1.2 Key findings from this study: .....................................................................................4 1.3 Key recommendations: .............................................................................................5 1.4 Ecological actions overview ......................................................................................7 2 Data availability on Swiss artificial lighting .......................................................................9 2.1 Summary ..................................................................................................................9 2.2 Introduction and background ..................................................................................10 2.3 Review and analysis of Swiss lighting data.............................................................10 2.3.1 VIIRS DNB (satellite mounted sensor).............................................................10 2.3.2 ISS images ......................................................................................................12 2.3.3 Street lamp inventories ....................................................................................18