The Role of Non-Native Plants in the Integration of Non-Native Phytophagous Invertebrates in Native Food Webs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Quercus Cerris
Quercus cerris Quercus cerris in Europe: distribution, habitat, usage and threats D. de Rigo, C. M. Enescu, T. Houston Durrant, G. Caudullo Turkey oak (Quercus cerris L.) is a deciduous tree native to southern Europe and Asia Minor, and a dominant species in the mixed forests of the Mediterranean basin. Turkey oak is a representative of section Cerris, a particular section within the genus Quercus which includes species for which the maturation of acorns occurs in the second year. Quercus cerris L., commonly known as Turkey oak, is a large fast-growing deciduous tree species growing to 40 m tall with 1 Frequency a trunk up to 1.5-2 m diameter , with a well-developed root < 25% system2. It can live for around 120-150 years3. The bark is 25% - 50% 50% - 75% mauve-grey and deeply furrowed with reddish-brown or orange > 75% bark fissures4, 5. Compared with other common oak species, e.g. Chorology Native sessile oak (Quercus petraea) and pedunculate oak (Quercus Introduced robur), the wood is inferior, and only useful for rough work such as shuttering or fuelwood1. The leaves are dark green above and grey-felted underneath6; they are variable in size and shape but are normally 9-12 cm long and 3-5 cm wide, with 7-9 pairs of triangular lobes6. The leaves turn yellow to gold in late autumn and drop off or persist in the crown until the next spring, especially on young trees3. The twigs are long and pubescent, grey or olive-green, with lenticels. The buds, which are concentrated Large shade tree in agricultural area near Altamura (Bari, South Italy). -

The Dorset Heath 2013 So Once Again You Have Me As Editor
NewsletterThe ofD theo Dorsetrset Flora H eGroupath 201 4 Chairman and VC9 Recorder Robin Walls; Secretary Laurence Taylor Editorial: John Newbould It would appear that the group had no complaints about the layout and content of the Dorset Heath 2013 so once again you have me as editor. The year was somewhat difficult for me as somehow, whenever I had to leave the room in Yorkshire Naturalists’ Union committee meetings in 2011, they managed to appoint me President for 2013 resulting in extra commitments in that county. During April 2013, Dorset hosted the National Forum for Biological Recording’s annual conference at the R.N.L.I. College at Poole. What a fabulous conference venue and the overnight accommodation was excellent. NFBR then joined Dorset naturalists with a joint meeting based at Studland helping to survey for the Cyril Diver project. Once again, duties took me away as I seem to be the conference administrator. The Flora Group had an interesting year, with variable numbers at field meetings. Never-the-less some important recording has been achieved including members engaging with recording bryophytes for the first time, one meeting to record fungi near Hardy’s Cottage, which thanks to the expertise of Bryan Edwards was very successful. We also had a few members try their hand at lichen recording In June 2014, I have been tasked by the Linnean Society to organise their annual field trip, which will be in June starting with a day on Portland and Chesil on the Saturday with Ballard Down and Studland on the Sunday. -

Harmful Non-Indigenous Species in the United States
Harmful Non-Indigenous Species in the United States September 1993 OTA-F-565 NTIS order #PB94-107679 GPO stock #052-003-01347-9 Recommended Citation: U.S. Congress, Office of Technology Assessment, Harmful Non-Indigenous Species in the United States, OTA-F-565 (Washington, DC: U.S. Government Printing Office, September 1993). For Sale by the U.S. Government Printing Office ii Superintendent of Documents, Mail Stop, SSOP. Washington, DC 20402-9328 ISBN O-1 6-042075-X Foreword on-indigenous species (NIS)-----those species found beyond their natural ranges—are part and parcel of the U.S. landscape. Many are highly beneficial. Almost all U.S. crops and domesticated animals, many sport fish and aquiculture species, numerous horticultural plants, and most biologicalN control organisms have origins outside the country. A large number of NIS, however, cause significant economic, environmental, and health damage. These harmful species are the focus of this study. The total number of harmful NIS and their cumulative impacts are creating a growing burden for the country. We cannot completely stop the tide of new harmful introductions. Perfect screening, detection, and control are technically impossible and will remain so for the foreseeable future. Nevertheless, the Federal and State policies designed to protect us from the worst species are not safeguarding our national interests in important areas. These conclusions have a number of policy implications. First, the Nation has no real national policy on harmful introductions; the current system is piecemeal, lacking adequate rigor and comprehensiveness. Second, many Federal and State statutes, regulations, and programs are not keeping pace with new and spreading non-indigenous pests. -
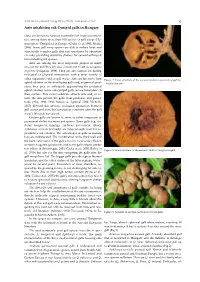
Ants Inhabiting Oak Cynipid Galls in Hungary
North-Western Journal of Zoology 2020, vol.16 (1) - Correspondence: Notes 95 Ants inhabiting oak Cynipid galls in Hungary Oaks are known to harbour extremely rich insect communi- ties, among them more than 100 species of gall wasps (Hy- menoptera: Cynipidae) in Europe (Csóka et al. 2005, Melika 2006). Some gall wasp species are able to induce large and structurally complex galls that can sometimes be abundant on oaks, providing attractive shelters for several arthropod taxa including ant species. Ants are among the most important players in many ecosystems and they are also considered to act as ecosystem engineers (Folgarait, 1998). They are also famous for having ecological or physical interactions with a great variety of other organisms, such as gall wasps. Ants are known to tend Figure 1. Inner structure of the asexual Andricus quercustozae gall in- aphid colonies on the developing galls and, as general pred- habited by ants. ators, they prey on arthropods approaching the protected aphid colonies. Some oak cynipid galls secrete honeydew on their surface. This sweet substrate attracts ants and, in re- turn, the ants protect the galls from predators and parasi- toids (Abe, 1988, 1992; Inouye & Agrawal 2004; Nicholls, 2017). Beyond this obvious ecological interaction between gall wasps and ants, this association continues after the gall wasp’s life cycle has ceased. Certain galls are known to serve as either temporary or permanent shelter for many ant species. Some galls (e.g. An- dricus hungaricus (Hartig), Andricus quercustozae (Bosc), Aphelonyx cerricola (Giraud)) are large enough even for re- productive ant colonies. The advantages of galls as nesting logs are multifaceted. -

The Structure of Cynipid Oak Galls: Patterns in the Evolution of an Extended Phenotype
The structure of cynipid oak galls: patterns in the evolution of an extended phenotype Graham N. Stone1* and James M. Cook2 1Department of Zoology, University of Oxford, South Parks Road, Oxford OX1 3PS, UK ([email protected]) 2Department of Biology, Imperial College, Silwood Park, Ascot, Berkshire SL5 7PY, UK Galls are highly specialized plant tissues whose development is induced by another organism. The most complex and diverse galls are those induced on oak trees by gallwasps (Hymenoptera: Cynipidae: Cyni- pini), each species inducing a characteristic gall structure. Debate continues over the possible adaptive signi¢cance of gall structural traits; some protect the gall inducer from attack by natural enemies, although the adaptive signi¢cance of others remains undemonstrated. Several gall traits are shared by groups of oak gallwasp species. It remains unknown whether shared traits represent (i) limited divergence from a shared ancestral gall form, or (ii) multiple cases of independent evolution. Here we map gall character states onto a molecular phylogeny of the oak cynipid genus Andricus, and demonstrate three features of the evolution of gall structure: (i) closely related species generally induce galls of similar structure; (ii) despite this general pattern, closely related species can induce markedly di¡erent galls; and (iii) several gall traits (the presence of many larval chambers in a single gall structure, surface resins, surface spines and internal air spaces) of demonstrated or suggested adaptive value to the gallwasp have evolved repeatedly. We discuss these results in the light of existing hypotheses on the adaptive signi¢cance of gall structure. Keywords: galls; Cynipidae; enemy-free space; extended phenotype; Andricus layers of woody or spongy tissue, complex air spaces within 1. -

Plant Health Карантин Растений
КАРАНТИН РАСТЕНИЙ СЕНТЯБРЬ НАУКА И ПРАКТИКА 3/25/2018 РУССКО-АНГЛИЙСКИЙ ЖУРНАЛ ВОЗБУДИТЕЛЬ ПРОЛИФЕРАЦИИ ЯБЛОНИ CANDIDATUS PHYTOPLASMA MALI стр. 4 ФИТОСАНИТАРНЫЙ РИСК РАСТИТЕЛЬНОЯДНЫХ КЛЕЩЕЙ (ARACHNIDA: ACARIFORMES) стр. 13 ИДЕНТИФИКАЦИЯ ВОЗБУДИТЕЛЯ РАКА КАРТОФЕЛЯ SYNCHYTRIUM ENDOBIOTICUM С ПРИМЕНЕНИЕМ МОЛЕКУЛЯРНЫХ МЕТОДОВ ДИАГНОСТИКИ стр. 27 ДИАГНОСТИКА НЕПОВИРУСА КОЛЬЦЕВОЙ ПЯТНИСТОСТИ ТОМАТА (ToRSV) МЕТОДОМ КЛАССИЧЕСКОЙ ПЦР стр. 41 CANDIDATUS PHYTOPLASMA MALI APPLE PROLIFERATION PATHOGEN page 9 PHYTOSANITARY RISK OF HERBIVOROUS MITES (ARACHNIDA: ACARIFORMES) page 20 IDENTIFICATION OF THE AGENT OF POTATO WART DISEASE SYNCHYTRIUM ENDOBIOTICUM USING MOLECULAR DIAGNOSTIC METHODS page 35 TOMATO RINGSPOT VIRUS DIAGNOSIS (ToRSV) USING CONVENTIONAL PCR page 46 RUSSIAN-ENGLISH JOURNAL PLANT HEALTH SEPTEMBER ISSN 2306-9767 ISSN RESEARCH AND PRACTICE 3/25/2018 «КАРАНТИН РАСТЕНИЙ. НАУКА И ПРАКТИКА» ДВУЯЗЫЧНЫЙ НАУЧНЫЙ ЖУРНАЛ №3 (25) 2018 г. Главный редактор: Мартин Уорд — РЕДАКЦИЯ: А.Я. Сапожников, Генеральный директор ЕОКЗР Волкова Е.М. — кандидат директор ФГБУ «ВНИИКР» биологических наук, Ханну Кукконен — директор заведующая лабораторией Шеф-редактор: подразделения фитосанитарного сорных растений Светлана Зиновьева, надзора, EVIRA (Финляндия) начальник отдела по связям Волков О.Г. — начальник с общественностью Сагитов А.О. — доктор отдела биометода и СМИ ФГБУ «ВНИИКР» биологических наук, Кулинич О.А. — доктор Генеральный директор ТОО биологических наук, Выпускающий редактор: «Казахский НИИ защиты начальник отдела лесного карантина Ольга Лесных и карантина растений» e-mail: [email protected] Приходько Ю.Н. — кандидат сельскохозяйственных наук, Сорока С.В. — кандидат Редакционная коллегия начальник научно-методического сельскохозяйственных наук, журнала «Карантин растений. отдела фитопатологии директор РУП «Институт Наука и практика»: защиты растений» НАН Скрипка О.В. — кандидат Швабаускене Ю.А. — заместитель Республики Беларусь биологических наук, ведущий Руководителя Россельхознадзора научный сотрудник лаборатории Джалилов Ф.С. -

National Oak Gall Wasp Survey
ational Oak Gall Wasp Survey – mapping with parabiologists in Finland Bess Hardwick Table of Contents 1. Introduction ................................................................................................................. 2 1.1. Parabiologists in data collecting ............................................................................. 2 1.2. Oak cynipid gall wasps .......................................................................................... 3 1.3. Motivations and objectives .................................................................................... 4 2. Material and methods ................................................................................................ 5 2.1. The volunteers ........................................................................................................ 5 2.2. Sampling ................................................................................................................. 6 2.3. Processing of samples ............................................................................................ 7 2.4. Data selection ........................................................................................................ 7 2.5. Statistical analyses ................................................................................................. 9 3. Results ....................................................................................................................... 10 3.1. Sampling success ................................................................................................. -

The Population Biology of Oak Gall Wasps (Hymenoptera:Cynipidae)
5 Nov 2001 10:11 AR AR147-21.tex AR147-21.SGM ARv2(2001/05/10) P1: GSR Annu. Rev. Entomol. 2002. 47:633–68 Copyright c 2002 by Annual Reviews. All rights reserved THE POPULATION BIOLOGY OF OAK GALL WASPS (HYMENOPTERA:CYNIPIDAE) Graham N. Stone,1 Karsten Schonrogge,¨ 2 Rachel J. Atkinson,3 David Bellido,4 and Juli Pujade-Villar4 1Institute of Cell, Animal, and Population Biology, University of Edinburgh, The King’s Buildings, West Mains Road, Edinburgh EH9 3JT, United Kingdom; e-mail: [email protected] 2Center of Ecology and Hydrology, CEH Dorset, Winfrith Technology Center, Winfrith Newburgh, Dorchester, Dorset DT2 8ZD, United Kingdom; e-mail: [email protected] 3Center for Conservation Science, Department of Biology, University of Stirling, Stirling FK9 4LA, United Kingdom; e-mail: [email protected] 4Departamento de Biologia Animal, Facultat de Biologia, Universitat de Barcelona, Avenida Diagonal 645, 08028 Barcelona, Spain; e-mail: [email protected] Key Words cyclical parthenogenesis, host alternation, food web, parasitoid, population dynamics ■ Abstract Oak gall wasps (Hymenoptera: Cynipidae, Cynipini) are characterized by possession of complex cyclically parthenogenetic life cycles and the ability to induce a wide diversity of highly complex species- and generation-specific galls on oaks and other Fagaceae. The galls support species-rich, closed communities of inquilines and parasitoids that have become a model system in community ecology. We review recent advances in the ecology of oak cynipids, with particular emphasis on life cycle characteristics and the dynamics of the interactions between host plants, gall wasps, and natural enemies. We assess the importance of gall traits in structuring oak cynipid communities and summarize the evidence for bottom-up and top-down effects across trophic levels. -

48 European Invertebrate Survey Nederland
issn 0169 - 2402 februari 2009 48 european invertebrate survey nieuwsbrief nederland 2 Nieuwsbrief European Invertebrate Survey – Nederland, 48 (2009) NIEUWSBRIEF van de EUROPEAN INVERTEBRATE SURVEY – NEDERLAND Nummer 48 - februari 2009 Contactorgaan voor de medewerkers van de Van de redactie werkgroepen van de European Invertebrate Survey – Nederland Deze extra nieuwsbrief, in kleur uitgegeven, is geheel gewijd aan het EIS-jubileum. De lezingen die Menno Schilthuizen en Informatie: Matthijs Schouten op de jubileumdag hebben gegeven kunt u Bureau EIS-Nederland, hier nog eens nalezen. Postbus 9517, 2300 RA Leiden tel. 071-5687670 / fax 071-5687666 Verder sluiten we de succesvolle inventarisatie van Naturalis- e-mail [email protected] terrein af. Het totaal aantal van 1569 soorten is al indrukwek- website www.naturalis.nl/eis kend, de grote hoeveelheid bijzonderheden is nog verbazing- wekkender. Het lijkt er op dat half-verwaarloosde terreinen in Wordt aan medewerkers gratis toegezonden. de stad een paradijs zijn voor schildwespen. Op deze plek wil ik alle personen bedanken die op een of andere manier hebben bijgedragen aan de soortenlijst. Tevens worden de fotografen Redactie: John T. Smit & Roy Kleukers bedankt voor het ter beschikking stellen van hun foto’s. Bij de soortenlijst worden zij met name genoemd, de beelden van de jubileumdag zijn voornamelijk van Berry van der Hoorn © copyright 2009 Stichting European Invertebrate Survey (Naturalis) en EIS-medewerkers. – Nederland, Leiden. Niets in deze uitgave mag worden vermenigvuldigd en/of openbaar Na zo’n mal jubileum van 33,3 jaar is het natuurlijk de vraag gemaakt door middel van fotokopie, microfilm of welke andere wijze wanneer het volgende feestje zal plaatsvinden. -

Butlleti 71.P65
Butll. Inst. Cat. Hist. Nat., 71: 83-95. 2003 ISSN: 1133-6889 GEA, FLORA ET FAUNA The life cycle of Andricus hispanicus (Hartig, 1856) n. stat., a sibling species of A. kollari (Hartig, 1843) (Hymenoptera: Cynipidae) Juli Pujade-Villar*, Roger Folliot** & David Bellido* Rebut: 28.07.03 Acceptat: 01.12.03 Abstract and so we consider A. mayeti and A. niger to be junior synonyms of A. hispanicus. Finally, possible causes of the speciation of A. kollari and The marble gallwasp, Andricus kollari, common A. hispanicus are discussed. and widespread in the Western Palaeartic, is known for the conspicuous globular galls caused by the asexual generations on the buds of several KEY WORDS: Cynipidae, Andricus, A. kollari, A. oak species. The sexual form known hitherto, hispanicus, biological cycle, sibling species, formerly named Andricus circulans, makes small sexual form, speciation, distribution, morphology, gregarious galls on the buds of Turkey oak, A. mayeti, A. burgundus. Quercus cerris; this oak, however, is absent from the Iberian Peninsula, where on the other hand the cork oak, Q. suber, is present. Recent genetic studies show the presence of two different Resum populations or races with distribution patterns si- milar to those of Q. cerris and Q. suber. We present new biological and morphological Cicle biològic d’Andricus hispanicus (Hartig, evidence supporting the presence of a sibling 1856) una espècie bessona d’A. kollari (Hartig, species of A. kollari in the western part of its 1843) (Hymenoptera: Cynipidae) range (the Iberian Peninsula, southern France and North Africa), Andricus hispanicus n. stat.. Biological and morphological differences separating these Andricus kollari és una espècie molt comuna dis- two species from other closely related ones are tribuida a l’oest del paleartic coneguda per la given and the new sexual form is described for the gal·la globular i relativament gran de la generació first time. -

Fossil Oak Galls Preserve Ancient Multitrophic Interactions
Edinburgh Research Explorer Fossil oak galls preserve ancient multitrophic interactions Citation for published version: Stone, GN, van der Ham, RWJM & Brewer, JG 2008, 'Fossil oak galls preserve ancient multitrophic interactions', Proceedings of the Royal Society B-Biological Sciences, vol. 275, no. 1648, pp. 2213-2219. https://doi.org/10.1098/rspb.2008.0494 Digital Object Identifier (DOI): 10.1098/rspb.2008.0494 Link: Link to publication record in Edinburgh Research Explorer Document Version: Publisher's PDF, also known as Version of record Published In: Proceedings of the Royal Society B-Biological Sciences Publisher Rights Statement: Free in PMC. General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 01. Oct. 2021 Proc. R. Soc. B (2008) 275, 2213–2219 doi:10.1098/rspb.2008.0494 Published online 17 June 2008 Fossil oak galls preserve ancient multitrophic interactions Graham N. Stone1,*, Raymond W. J. M. van der Ham2 and Jan G. Brewer3 1Institute of Evolutionary Biology, University of Edinburgh, West Mains Road, Edinburgh EH9 3JT, UK 2Nationaal Herbarium Nederland, Universiteit Leiden, PO Box 9514, 2300 RA Leiden, The Netherlands 3Hogebroeksweg 32, 8102 RK Raalte, The Netherlands Trace fossils of insect feeding have contributed substantially to our understanding of the evolution of insect–plant interactions. -

Newsletter Number 29 September 1992 New Zealand Botanical Society Newsletter Number 29 September 1992
NEW ZEALAND BOTANICAL SOCIETY NEWSLETTER NUMBER 29 SEPTEMBER 1992 NEW ZEALAND BOTANICAL SOCIETY NEWSLETTER NUMBER 29 SEPTEMBER 1992 CONTENTS News NZ Bot Soc News Call for nominations 2 New Zealand Threatened Indigenous Vascular Plant List .2 Regional Bot Soc News Auckland 5 Canterbury 6 Nelson 6 Rotorua 7 Waikato 7 Wellington 8 Obituary Margot Forde 8 Other News Distinguished New Zealand Scientist turns 100 9 Government Science structures reorganised 10 New Department consolidates Marine Science strengths 10 Notes and Reports Plant records Conservation status of titirangi (Hebe speciosa) 11 Senecio sterquilinus Ornduff in the Wellington Ecological District ....... 16 Trip reports Ecological Forum Excursion to South Patagonia and Tierra del Fuego (2) .... 17 Tangihua Fungal Foray, 20-24 May 1992 19 Biography/Bibliography Biographical Notes (6) Peter Goyen, an addition 20 Biographical Notes (7) Joshua Rutland 20 New Zealand Botanists and Fellowships of the Royal Society 22 Forthcoming Meetings/Conferences Lichen Techniques Workshop 22 Forthcoming Trips/Tours Seventh New Zealand Fungal Foray 22 Publications Checklist of New Zealand lichens 23 The mosses of New Zealand, special offer 24 Book review An illustrated guide to fungi on wood in New Zealand 25 Letters to the Editor New Zealand Botanical Society President: Dr Eric Godley Secretary/Treasurer: Anthony Wright Committee: Sarah Beadel, Ewen Cameron, Colin Webb, Carol West Address: New Zealand Botanical Society C/- Auckland Institute & Museum Private Bag 92018 AUCKLAND Subscriptions The 1992 ordinary and institutional subs are $14 (reduced to $10 if paid by the due date on the subscription invoice). The 1992 student sub, available to full-time students, is $7 (reduced to $5 if paid by the due date on the subscription invoice).