ESM1 As a Marker of Macrotrabecular-Massive Hepatocellular Carcinoma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Environmental Influences on Endothelial Gene Expression
ENDOTHELIAL CELL GENE EXPRESSION John Matthew Jeff Herbert Supervisors: Prof. Roy Bicknell and Dr. Victoria Heath PhD thesis University of Birmingham August 2012 University of Birmingham Research Archive e-theses repository This unpublished thesis/dissertation is copyright of the author and/or third parties. The intellectual property rights of the author or third parties in respect of this work are as defined by The Copyright Designs and Patents Act 1988 or as modified by any successor legislation. Any use made of information contained in this thesis/dissertation must be in accordance with that legislation and must be properly acknowledged. Further distribution or reproduction in any format is prohibited without the permission of the copyright holder. ABSTRACT Tumour angiogenesis is a vital process in the pathology of tumour development and metastasis. Targeting markers of tumour endothelium provide a means of targeted destruction of a tumours oxygen and nutrient supply via destruction of tumour vasculature, which in turn ultimately leads to beneficial consequences to patients. Although current anti -angiogenic and vascular targeting strategies help patients, more potently in combination with chemo therapy, there is still a need for more tumour endothelial marker discoveries as current treatments have cardiovascular and other side effects. For the first time, the analyses of in-vivo biotinylation of an embryonic system is performed to obtain putative vascular targets. Also for the first time, deep sequencing is applied to freshly isolated tumour and normal endothelial cells from lung, colon and bladder tissues for the identification of pan-vascular-targets. Integration of the proteomic, deep sequencing, public cDNA libraries and microarrays, delivers 5,892 putative vascular targets to the science community. -

Inducers of the Endothelial Cell Barrier Identified Through Chemogenomic Screening in Genome-Edited Hpsc-Endothelial Cells
Inducers of the endothelial cell barrier identified through chemogenomic screening in genome-edited hPSC-endothelial cells Filip Roudnickya,1, Jitao David Zhang (张继涛)b,1,2, Bo Kyoung Kimc, Nikhil J. Pandyab, Yanjun Lana, Lisa Sach-Peltasond, Heloise Ragellec, Pamela Strassburgerc, Sabine Gruenerc, Mirjana Lazendicc, Sabine Uhlesc, Franco Revelantc, Oliv Eidamd, Gregor Sturmb, Verena Kueppersc, Klaus Christensena, Leonard D. Goldsteine, Manuel Tzourosb, Balazs Banfaib, Zora Modrusane, Martin Grafa, Christoph Patscha, Mark Burcina, Claas A. Meyera,3, Peter D. Westenskowc,2,3, and Chad A. Cowanf,g,h,2,3 aTherapeutic Modalities, Pharmaceutical Research and Early Development, Roche Innovation Center Basel, F. Hoffmann-La Roche Ltd., CH-4070 Basel, Switzerland; bPharmaceutical Sciences, Pharmaceutical Research and Early Development, Roche Innovation Center Basel, F. Hoffmann-La Roche Ltd., CH-4070 Basel, Switzerland; cOcular Technologies, Immunology, Infectious Diseases and Ophthalmology, Pharmaceutical Research and Early Development, Roche Innovation Center Basel, F. Hoffmann-La Roche Ltd., CH-4070 Basel, Switzerland; dPharma Research and Early Development Informatics, Pharmaceutical Research and Early Development, Roche Innovation Center Basel, F. Hoffmann-La Roche Ltd., CH-4070 Basel, Switzerland; eMolecular Biology Department, Genentech Inc., South San Francisco, CA 94080; fDivision of Cardiology, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02215; gDepartment of Stem Cell and Regenerative -

Peripheral Nerve Single-Cell Analysis Identifies Mesenchymal Ligands That Promote Axonal Growth
Research Article: New Research Development Peripheral Nerve Single-Cell Analysis Identifies Mesenchymal Ligands that Promote Axonal Growth Jeremy S. Toma,1 Konstantina Karamboulas,1,ª Matthew J. Carr,1,2,ª Adelaida Kolaj,1,3 Scott A. Yuzwa,1 Neemat Mahmud,1,3 Mekayla A. Storer,1 David R. Kaplan,1,2,4 and Freda D. Miller1,2,3,4 https://doi.org/10.1523/ENEURO.0066-20.2020 1Program in Neurosciences and Mental Health, Hospital for Sick Children, 555 University Avenue, Toronto, Ontario M5G 1X8, Canada, 2Institute of Medical Sciences University of Toronto, Toronto, Ontario M5G 1A8, Canada, 3Department of Physiology, University of Toronto, Toronto, Ontario M5G 1A8, Canada, and 4Department of Molecular Genetics, University of Toronto, Toronto, Ontario M5G 1A8, Canada Abstract Peripheral nerves provide a supportive growth environment for developing and regenerating axons and are es- sential for maintenance and repair of many non-neural tissues. This capacity has largely been ascribed to paracrine factors secreted by nerve-resident Schwann cells. Here, we used single-cell transcriptional profiling to identify ligands made by different injured rodent nerve cell types and have combined this with cell-surface mass spectrometry to computationally model potential paracrine interactions with peripheral neurons. These analyses show that peripheral nerves make many ligands predicted to act on peripheral and CNS neurons, in- cluding known and previously uncharacterized ligands. While Schwann cells are an important ligand source within injured nerves, more than half of the predicted ligands are made by nerve-resident mesenchymal cells, including the endoneurial cells most closely associated with peripheral axons. At least three of these mesen- chymal ligands, ANGPT1, CCL11, and VEGFC, promote growth when locally applied on sympathetic axons. -
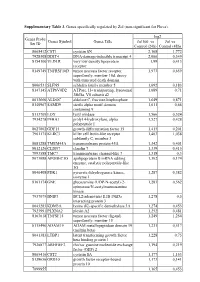
Supplementary Table 3. Genes Specifically Regulated by Zol (Non-Significant for Fluva)
Supplementary Table 3. Genes specifically regulated by Zol (non-significant for Fluva). log2 Genes Probe Genes Symbol Genes Title Zol100 vs Zol vs Set ID Control (24h) Control (48h) 8065412 CST1 cystatin SN 2,168 1,772 7928308 DDIT4 DNA-damage-inducible transcript 4 2,066 0,349 8154100 VLDLR very low density lipoprotein 1,99 0,413 receptor 8149749 TNFRSF10D tumor necrosis factor receptor 1,973 0,659 superfamily, member 10d, decoy with truncated death domain 8006531 SLFN5 schlafen family member 5 1,692 0,183 8147145 ATP6V0D2 ATPase, H+ transporting, lysosomal 1,689 0,71 38kDa, V0 subunit d2 8013660 ALDOC aldolase C, fructose-bisphosphate 1,649 0,871 8140967 SAMD9 sterile alpha motif domain 1,611 0,66 containing 9 8113709 LOX lysyl oxidase 1,566 0,524 7934278 P4HA1 prolyl 4-hydroxylase, alpha 1,527 0,428 polypeptide I 8027002 GDF15 growth differentiation factor 15 1,415 0,201 7961175 KLRC3 killer cell lectin-like receptor 1,403 1,038 subfamily C, member 3 8081288 TMEM45A transmembrane protein 45A 1,342 0,401 8012126 CLDN7 claudin 7 1,339 0,415 7993588 TMC7 transmembrane channel-like 7 1,318 0,3 8073088 APOBEC3G apolipoprotein B mRNA editing 1,302 0,174 enzyme, catalytic polypeptide-like 3G 8046408 PDK1 pyruvate dehydrogenase kinase, 1,287 0,382 isozyme 1 8161174 GNE glucosamine (UDP-N-acetyl)-2- 1,283 0,562 epimerase/N-acetylmannosamine kinase 7937079 BNIP3 BCL2/adenovirus E1B 19kDa 1,278 0,5 interacting protein 3 8043283 KDM3A lysine (K)-specific demethylase 3A 1,274 0,453 7923991 PLXNA2 plexin A2 1,252 0,481 8163618 TNFSF15 tumor necrosis -

ESM1 As a Marker of Macrotrabecular-Massive Hepatocellular Carcinoma
Author Manuscript Published OnlineFirst on July 29, 2019; DOI: 10.1158/1078-0432.CCR-19-0859 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. ESM1 as a marker of macrotrabecular-massive hepatocellular carcinoma. Julien Calderaro1,2,3, Léa Meunier4, Cong Trung Nguyen2,3, Marouane Boubaya5, Stefano Caruso4, Alain Luciani2,3,6, Giuliana Amaddeo2,3,7, Hélène Regnault7, Jean-Charles Nault4,8,9 , Justine Cohen1,2,Frédéric Oberti10, Sophie Michalak11, Mohamed Bouattour12, Valérie Vilgrain18, Georges Philippe Pageaux14, Jeanne Ramos15, Nathalie Barget16, Boris Guiu17, Valérie Paradis13, Christophe Aubé19, Alexis Laurent20, Jean-Michel Pawlotsky2,3,22, Nathalie Ganne-Carrié4,8,9, Jessica Zucman-Rossi4,22,23, Olivier Seror24, Marianne Ziol4,9,25. 1. Assistance Publique-Hôpitaux de Paris, Département Pathologie, CHU Henri Mondor, F-94000 Créteil, France. 2. Université Paris-Est Créteil, Faculté de Médecine, Créteil, France. 3. Inserm, U955, Team 18, Créteil, France. 4. INSERM UMR-1162, génomique fonctionnelle des tumeurs solides, 75010, Paris, France 5. Unité de Recherche Clinique, AP-HP, Hôpital Universitaire Avicenne, Bobigny, France. 6. Assistance Publique-Hôpitaux de Paris, Service de Radiologie, CHU Henri Mondor, F-94000 Créteil, France 7. Assistance Publique-Hôpitaux de Paris, Service d'Hépatologie, CHU Henri Mondor, F-94000 Créteil, France. 8. Service d’Hépatologie, Groupe hospitalier Paris-Seine-Saint Denis, Hôpital Jean Verdier, AP-HP, 93143 Bondy, France, 9. Université Paris 13, Sorbonne Paris-Cité, Bobigny, France 10. Hépato-gastroentérologie et oncologie digestive, Centre Hospitalier Universitaire d'Angers, France. 11. Service d'Anatomie et de Cytologie Pathologiques, Centre Hospitalier Universitaire d'Angers, France. 12. Assistance Publique-Hôpitaux de Paris, Service d’Oncologie Digestive, Hôpital Universitaire Beaujon, France. -

Impact of Gene Expression Profiling Tests on Breast Cancer Outcomes
Evidence Report/Technology Assessment Number 160 Impact of Gene Expression Profiling Tests on Breast Cancer Outcomes Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 540 Gaither Road Rockville, MD 20850 www.ahrq.gov Contract No. 290-02-0018 Prepared by: The Johns Hopkins University Evidence-based Practice Center, Baltimore, MD Investigators Luigi Marchionni, M.D., Ph.D. Renee F. Wilson, M.Sc. Spyridon S. Marinopoulos, M.D., M.B.A. Antonio C. Wolff, M.D. Giovanni Parmigiani, M.D. Eric B. Bass, M.D., M.P.H. Steven N. Goodman, M.D., M.H.S., Ph.D. AHRQ Publication No. 08-E002 January 2008 This report is based on research conducted by the Johns Hopkins University Evidence-based Practice Center (EPC) under contract to the Agency for Healthcare Research and Quality (AHRQ), Rockville, MD (Contract No. 290-02-0018). The findings and conclusions in this document are those of the author(s), who are responsible for its content, and do not necessarily represent the views of AHRQ. No statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services. The information in this report is intended to help clinicians, employers, policymakers, and others make informed decisions about the provision of health care services. This report is intended as a reference and not as a substitute for clinical judgment. This report may be used, in whole or in part, as the basis for the development of clinical practice guidelines and other quality enhancement tools, or as a basis for reimbursement and coverage policies. -
Download Validation Data
PrimePCR™Assay Validation Report Gene Information Gene Name endothelial cell-specific molecule 1 Gene Symbol ESM1 Organism Human Gene Summary This gene encodes a secreted protein which is mainly expressed in the endothelial cells in human lung and kidney tissues. The expression of this gene is regulated by cytokines suggesting that it may play a role in endothelium-dependent pathological disorders. The transcript contains multiple polyadenylation and mRNA instability signals. Two transcript variants encoding different isoforms have been found for this gene. Gene Aliases endocan RefSeq Accession No. NC_000005.9, NT_006713.15 UniGene ID Hs.129944 Ensembl Gene ID ENSG00000164283 Entrez Gene ID 11082 Assay Information Unique Assay ID qHsaCEP0040414 Assay Type Probe - Validation information is for the primer pair using SYBR® Green detection Detected Coding Transcript(s) ENST00000381405, ENST00000381403, ENST00000601836 Amplicon Context Sequence GGCTGACACCTCAGCCCCGGGCCACACTTCATGCCATCCATGCCTGAGACTGTG CGGTAGCAAGTTTCTCCCCGCCCTGCAGCGCACACTCGGCAGCAGCCACAGTC GTCGAGCACTGTCCTCTTGCAGCGCG Amplicon Length (bp) 103 Chromosome Location 5:54281092-54281224 Assay Design Exonic Purification Desalted Validation Results Efficiency (%) 98 R2 0.9994 cDNA Cq 23.35 cDNA Tm (Celsius) 87.5 Page 1/5 PrimePCR™Assay Validation Report gDNA Cq 23.86 Specificity (%) 100 Information to assist with data interpretation is provided at the end of this report. Page 2/5 PrimePCR™Assay Validation Report ESM1, Human Amplification Plot Amplification of cDNA generated from 25 ng of -

Recombinant Mouse Endothelial Cell-Specific Molecule /Endocan/ESM-1 (C-6His)
9853 Pacific Heights Blvd. Suite D. San Diego, CA 92121, USA Tel: 858-263-4982 Email: [email protected] 32-8716: Recombinant Mouse Endothelial Cell-Specific Molecule /Endocan/ESM-1 (C-6His) Gene : Esm1 Gene ID : 71690 Uniprot ID : Q9QYY7 Description Source: Human Cells. MW :19kD. Recombinant Mouse ESM-1 is produced by our Mammalian expression system and the target gene encoding Trp20-Arg184 is expressed with a 6His tag at the C-terminus. Endothelial cell-specific molecule–1 (ESM-1)—so-called endocan—is a novel endothelium derived soluble dermatan sulfate proteoglycan (PG) that is constitutively expressed by endothelial cells in lungs and kidneys and can be detected in human blood. It is encoded by the ESM1 gene. The expression of this gene is regulated by several cytokines and growth factors, such as vascular endothelial growth factor. Inflammatory cytokines, such as interleukin (IL)-1 beta and tumor necrosis factor (TNF)-a, stimulate the upregulation of endocan mRNA and the secretion of endocan from endothelial cells. The binding of circulating endocan to leukocyte ligand for ICAM-1—Lymphocyte Function-associated Antigen-1 (LFA-1) and to leukocyte ligand for VCAM-1—Very Late Antigen-4 (VLA-4) is important in leukocyte adhesion and interaction with activated endothelium. Endocan is a key player in the regulation of major processes such as cell adhesion in inflammatory disorders and tumor progression. Product Info Amount : 10 µg / 50 µg Content : Lyophilized from a 0.2 µm filtered solution of PBS, pH7.4. Lyophilized protein should be stored at -20°C, though stable at room temperature for 3 weeks. -

5Q11.2 Deletion in a Patient with Tracheal Agenesis
European Journal of Human Genetics (2010) 18, 1265–1268 & 2010 Macmillan Publishers Limited All rights reserved 1018-4813/10 www.nature.com/ejhg SHORT REPORT 5q11.2 deletion in a patient with tracheal agenesis Elisabeth M de Jong1,2, Hannie Douben1, Bert H Eussen1, Janine F Felix2, Marja W Wessels1, Pino J Poddighe1, Peter GJ Nikkels3, Ronald R de Krijger4, Dick Tibboel2 and Annelies de Klein*,1 Tracheal agenesis (TA) is a rare congenital anomaly of the respiratory tract. Many patients have associated anomalies, suggesting a syndromal phenotype. In a cohort of 12 patients, we aimed to detect copy number variations. In addition to routine cytogenetic analysis, we applied oligonucleotide array comparative genomic hybridization. Our patient cohort showed various copy number variations, of which many were parentally inherited variants. One patient had, in addition to an inherited 16p12.1 deletion, a 3.6 Mb deletion on chromosomal locus 5q11.2. This patient had a syndromic phenotype, including vertebral, anal, cardiovascular and tracheo-oesophageal associated anomalies, and other foregut-related anomalies, such as cartilage rings in the oesophagus and an aberrant right bronchus. No common deletions or duplications are found in our cohort, suggesting that TA is a genetically heterogeneous disorder. European Journal of Human Genetics (2010) 18, 1265–1268; doi:10.1038/ejhg.2010.84; published online 16 June 2010 Keywords: tracheal agenesis; array comparative genomic hybridization; 5q11; deletion; VACTERL; TACRD INTRODUCTION and included at the department of Pathology, University Medical Centre Tracheal agenesis (TA) is a rare congenital anomaly in which the Utrecht. The medical records and pathology records were reviewed. -

Esm1 and Stc1 As Angiogenic Factors Responsible for Protective Actions of Adipose-Derived Stem Cell Sheets on Chronic Heart Failure After Rat Myocardial Infarction
Advance Publication Circulation Journal ORIGINAL ARTICLE doi: 10.1253/circj.CJ-20-0877 Esm1 and Stc1 as Angiogenic Factors Responsible for Protective Actions of Adipose-Derived Stem Cell Sheets on Chronic Heart Failure After Rat Myocardial Infarction Mai Watanabe, BSc; Hiromu Horie, MD; Yasutaka Kurata, MD, PhD; Yumiko Inoue, BSc; Tomomi Notsu, BSc; Takayuki Wakimizu, BSc; Maya Adachi, BSc; Kenshiro Yamamoto, BSc; Kumi Morikawa, PhD; Masanari Kuwabara, MD, PhD; Takuki Sakaguchi, MD; Takayuki Morisaki, MD, PhD; Junichiro Miake, MD, PhD; Motonobu Nishimura, MD, PhD; Motokazu Tsuneto, PhD; Yasuaki Shirayoshi, PhD; Shin Ito, MD, PhD; Masafumi Kitakaze, MD, PhD; Haruaki Ninomiya, MD, PhD; Kazuhiro Yamamoto, MD, PhD; Ichiro Hisatome, MD, PhD Background: Although adipose-derived stem cell (ADSC) sheets improve the cardiac function after myocardial infarction (MI), underlying mechanisms remain to be elucidated. The aim of this study was to determine the fate of transplanted ADSC sheets and candidate angiogenic factors released from ADSCs for their cardiac protective actions. Methods and Results: MI was induced by ligation of the left anterior descending coronary artery. Sheets of transgenic (Tg)-ADSCs expressing green fluorescence protein (GFP) and luciferase or wild-type (WT)-ADSCs were transplanted 1 week after MI. Both WT- and Tg-ADSC sheets improved cardiac functions evaluated by echocardiography at 3 and 5 weeks after MI. Histological examination at 5 weeks after MI demonstrated that either sheet suppressed fibrosis and increased vasculogenesis. Luciferase signals from Tg-ADSC sheets were detected at 1 and 2 weeks, but not at 4 weeks, after transplantation. RNA sequencing of PKH (yellow-orange fluorescent dye with long aliphatic tails)-labeled Tg-ADSCs identified mRNAs of 4 molecules related to angiogenesis, including those of Esm1 and Stc1 that increased under hypoxia. -

Data-Driven and Knowledge-Driven Computational Models of Angiogenesis in Application to Peripheral Arterial Disease
DATA-DRIVEN AND KNOWLEDGE-DRIVEN COMPUTATIONAL MODELS OF ANGIOGENESIS IN APPLICATION TO PERIPHERAL ARTERIAL DISEASE by Liang-Hui Chu A dissertation submitted to Johns Hopkins University in conformity with the requirements for the degree of Doctor of Philosophy Baltimore, Maryland March, 2015 © 2015 Liang-Hui Chu All Rights Reserved Abstract Angiogenesis, the formation of new blood vessels from pre-existing vessels, is involved in both physiological conditions (e.g. development, wound healing and exercise) and diseases (e.g. cancer, age-related macular degeneration, and ischemic diseases such as coronary artery disease and peripheral arterial disease). Peripheral arterial disease (PAD) affects approximately 8 to 12 million people in United States, especially those over the age of 50 and its prevalence is now comparable to that of coronary artery disease. To date, all clinical trials that includes stimulation of VEGF (vascular endothelial growth factor) and FGF (fibroblast growth factor) have failed. There is an unmet need to find novel genes and drug targets and predict potential therapeutics in PAD. We use the data-driven bioinformatic approach to identify angiogenesis-associated genes and predict new targets and repositioned drugs in PAD. We also formulate a mechanistic three- compartment model that includes the anti-angiogenic isoform VEGF165b. The thesis can serve as a framework for computational and experimental validations of novel drug targets and drugs in PAD. ii Acknowledgements I appreciate my advisor Dr. Aleksander S. Popel to guide my PhD studies for the five years at Johns Hopkins University. I also appreciate several professors on my thesis committee, Dr. Joel S. Bader, Dr. -

The Glomerular Transcriptome and a Predicted Protein–Protein Interaction Network
BASIC RESEARCH www.jasn.org The Glomerular Transcriptome and a Predicted Protein–Protein Interaction Network Liqun He,* Ying Sun,* Minoru Takemoto,* Jenny Norlin,* Karl Tryggvason,* Tore Samuelsson,† and Christer Betsholtz*‡ *Division of Matrix Biology, Department of Medical Biochemistry and Biophysics, and ‡Department of Medicine, Karolinska Institutet, Stockholm, and †Department of Medical Biochemistry, Go¨teborg University, Go¨teborg, Sweden ABSTRACT To increase our understanding of the molecular composition of the kidney glomerulus, we performed a meta-analysis of available glomerular transcriptional profiles made from mouse and man using five different methodologies. We generated a combined catalogue of glomerulus-enriched genes that emerged from these different sources and then used this to construct a predicted protein–protein interaction network in the glomerulus (GlomNet). The combined glomerulus-enriched gene catalogue provides the most comprehensive picture of the molecular composition of the glomerulus currently available, and GlomNet contributes an integrative systems biology approach to the understanding of glomerular signaling networks that operate during development, function, and disease. J Am Soc Nephrol 19: 260–268, 2008. doi: 10.1681/ASN.2007050588 Many kidney diseases and, importantly, approxi- nins have been shown to be mutated in Alport syn- mately two thirds of all cases of ESRD originate with drome and Pierson congenital nephrotic syndromes, glomerular disease. Most cases of glomerular disease respectively.11,12 Genetic studies in mice have further are caused by systemic disorders (e.g., diabetes, hyper- revealed genes and proteins of importance for glomer- tension, lupus, obesity) for which the molecular ulus development and function, such as podoca- pathogeneses of the glomerular complications are un- lyxin,13 CD2AP,14 NEPH1,15 FAT1,16 forkhead box known.