Personal Protective Equipment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evacuation Coordinator Handbook
EVACUATION COORDINATOR HANDBOOK EC Prepared by: Luella Ackerson, Risk Control Analyst DAS EGS Risk Management Revised 2017 - New 2013 Page 1 of 37 Page 2 of 37 TABLE OF CONTENTS Glossary 4 Resources 4 Site Emergency Coordinator (SEC) 5 Evacuation Coordinator (EC) 6 Basic Planning Information for SECs and ECs 7 Evacuation, Route and Assembly Area Summary 12 Emergency Roles of Other Entities 13 Assisting Individuals with Disabilities 15 Fire Emergencies: For SECs, ECs and Employees 21 Earthquakes: Two Levels of Risk 22 Earthquake Instructions for Office Workers 25 Hazardous Materials: Two Levels of Risk 27 Chemical Spill Outside of Building 29 Armed and Dangerous Intruders 31 Threat of Explosion: Two Levels of Risk 33 Extreme Events 35 Evacuation in Progress Sign 37 DAS East Evacuation Assembly Area Map A1 Capitol Mall Building Drills and Site Emergency Coordinators A2 Page 3 of 37 GLOSSARY ADA – Americans with Disability Act DAS – Department of Administrative Services EA – Evacuation Assistant EC – Evacuation Coordinator Emergency Responder – Local Fire Department, city, county or State Police Employee – State of Oregon worker Employer – State of Oregon SEC – Site Emergency Coordinator Statewide Emergency Entities – Fire Marshal, Emergency Management, ORNG, etc. RESOURCES Administrative Rule for Earthquake Preparedness Drills OAR 104-020 Hazards and Preparedness: Earthquakes Earthquakes and Other Natural Hazards in the Pacific Northwest Oregon Office of Emergency Management 9-1-1 Program Put Together an Emergency Kit Emergency Evacuation Planning Guide for People with Disabilities Page 4 of 37 SITE EMERGENCY COORDINATOR (SEC) The Site Emergency Coordinator (SEC) and one back-up SEC must be appointed for each office building. -

Biological Safety Standard Operating Procedure (SOP) SOP Title
IBSP005 Biosafety SOP template January 2021 Icahn School of Medicine at Mount Sinai Biological Safety Standard Operating Procedure (SOP) SOP Title SOP Number (version) IBC registration(s) Section 1. Laboratory-specific information Building/Room(s) Department/Institute SOP author LSO PI name PI signature Section 2. Biological hazard information Biological Agent1 RG2 BSL3 Potential Hazards Signs/Symptoms of Occupational Health ABSL4 Infection Requirements Section 3. Other hazards Sharps Hazards Other Biological Hazards Chemical Hazards Radiological Hazards Section 4. Personal Protective Equipment (PPE) Laboratory Coat Fluid-Resistant Gown Surgical Mask Gloves Shoe Covers N95 Respirator Eye Protection Fluid-Resistant Sleeves PAPR Other PPE 1 Specific strains of biological agents should be described in the corresponding IBC registration. 2 NIH Risk Groups: RG-1, RG-2, RG-3, or RG-4 3 Biological Safety Level: BSL-1, BSL-2, BSL-3, BSL-4 4 Animal Biological Safety Level: ABSL-1, ABSL-2, ABSL-3, ABSL-4 Page | 1 IBSP005 Biosafety SOP template January 2021 Section 5. Equipment and Engineering Controls Biological Safety Cabinet Centrifuge Aerosol-Generating Equipment Other equipment Section 6. Decontamination Disinfectant Contact Time Dilution Location Section 7. Waste Management/Disposal Steam Sterilizer Location: (Autoclave) Chemical Disinfectant(s) Location: Other Disinfectant(s) Location: Section 8. Transport Procedure(s) Page | 2 IBSP005 Biosafety SOP template January 2021 Section 9. Spill Response Procedure Section 10. Protocol Procedure Page | 3 IBSP005 Biosafety SOP template January 2021 Section 10. Protocol Procedure Continued (attach additional sheets if necessary) Page | 4 IBSP005 Biosafety SOP template January 2021 Section 11. Documentation of training and understanding. The Principal Investigator must ensure that all laboratory personnel receive training on the content of this SOP. -

Risk Assessment Guidance
Risk Assessment Guidance The assessor can assign values for the hazard severity (a) and likelihood of occurrence (b) (taking into account the frequency and duration of exposure) on a scale of 1 to 5, then multiply them together to give the rating band: Hazard Severity (a) Likelihood of Occurrence (b) 1 – Trivial (eg discomfort, slight bruising, self-help recovery) 1 – Remote (almost never) 2 – Minor (eg small cut, abrasion, basic first aid need) 2 – Unlikely (occurs rarely) 3 – Moderate (eg strain, sprain, incapacitation > 3 days) 3 – Possible (could occur, but uncommon) 4 – Serious (eg fracture, hospitalisation >24 hrs, incapacitation >4 4 – Likely (recurrent but not frequent) weeks) 5 – Very likely (occurs frequently) 5 – Fatal (single or multiple) The risk rating (high, medium or low) indicates the level of response required to be taken when designing the action plan. Trivial Minor Moderate Serious Fatal Rating Bands (a x b) Remote 1 2 3 4 5 LOW RISK MEDIUM RISK HIGH RISK (1 – 8) (9 - 12) (15 - 25) Unlikely 2 4 6 8 10 Continue, but Continue, but -STOP THE Possible review implement ACTIVITY- 3 6 9 12 15 periodically to additional Identify new ensure controls reasonably controls. Activity Likely remain effective practicable must not controls where 4 8 12 16 20 proceed until possible and risks are Very monitor regularly reduced to a low likely 5 10 15 20 25 or medium level 1 Risk Assessments There are a number of explanations needed in order to understand the process and the form used in this example: HAZARD: Anything that has the potential to cause harm. -

Permits-To-Work in the Process Industries
SYMPOSIUM SERIES NO. 151 # 2006 IChemE PERMITS-TO-WORK IN THE PROCESS INDUSTRIES John Gould Environmental Resources Management, Suite 8.01, 8 Exchange Quay, Manchester M5 3EJ; [email protected] The paper presents the collective results from a number of Safety Management System audits. The audit protocol is based on the Health and Safety Executive pub- lication ‘Successful health and safety management’ and takes into account formal (written) and informal procedures as well as their implementation. Focused on permit-to-work systems, these have shown a number of common failings. The most common failure in implementing a permit-to-work system is the issue of too many permits. However, the audit protocol considers the whole risk control system. The failure to ‘close’ the management loop with an effective regular review process is the largest obstacle to an effective permit system. INTRODUCTION ‘Permits save lives – give them proper attention’. This is a startling statement made by the Health and Safety Executive (HSE) in its free leaflet IND(G) 98 (Rev 3) PTW systems. The leaflet goes on to state that two thirds of all accidents in the chemical industry are main- tenance related, with the permit-to-work (PTW) failures being the largest single cause. Given these facts, it comes as no surprise that PTW systems are a key part in the provision of a safe working environment. Over the past four years Environmental Resources Management (ERM) has been auditing PTW systems as part of its key risk control systems audits. Numerous systems have been evaluated from a wide rage of industries, covering personal care products man- ufacturing to refinery operations. -

Occupational Safety and Health Admin., Labor § 1910.145
Occupational Safety and Health Admin., Labor § 1910.145 of the workers or their families, shall (i) Fire protection equipment and appa- be provided in connection with all food ratus. [Reserved] handling facilities. There shall be no (ii) Danger. Safety cans or other port- direct opening from living or sleeping able containers of flammable liquids quarters into a kitchen or dining hall. having a flash point at or below 80° F, (3) No person with any communicable table containers of flammable liquids disease shall be employed or permitted (open cup tester), excluding shipping to work in the preparation, cooking, containers, shall be painted red with serving, or other handling of food, food- some additional clearly visible identi- stuffs, or materials used therein, in fication either in the form of a yellow any kitchen or dining room operated in band around the can or the name of the connection with a camp or regularly contents conspicuously stenciled or used by persons living in a camp. painted on the can in yellow. Red (j) Insect and rodent control. Effective lights shall be provided at barricades measures shall be taken to prevent in- and at temporary obstructions, as spec- festation by and harborage of animal ified in ANSI Safety Code for Building or insect vectors or pests. Construction, A10.2±1944, which is in- (k) First aid. (1) Adequate first aid fa- corporated by reference as specified in cilities approved by a health authority § 1910.6. Danger signs shall be painted shall be maintained and made available red. in every labor camp for the emergency (iii) Stop. -
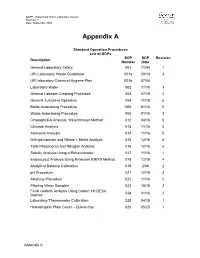
Lab Standard Operating Procedures (Sops)
QAPP –Watershed Watch Laboratory Assays Revision: 7 Date: September 2020 Appendix A Standard Operation Procedures List of SOPs SOP SOP Revision Description Number Date General Laboratory Safety 001 11/04 1 URI Laboratory Waste Guidebook 001a 09/13 3 URI laboratory Chemical Hygiene Plan 001b 07/04 Laboratory Water 002 11/16 3 General Labware Cleaning Procedure 003 07/19 4 General Autoclave Operation 004 10/18 6 Bottle Autoclaving Procedure 005 01/16 5 Waste Autoclaving Procedure 006 01/16 3 Chlorophyll-A Analysis, Welschmeyer Method 012 04/18 6 Chloride Analysis 013 11/16 5 Ammonia Analysis 014 11/16 5 Orthophosphate and Nitrate + Nitrite Analysis 015 12/16 5 Total Phosphorus and Nitrogen Analysis 016 12/16 5 Salinity Analysis Using a Refractometer 017 11/16 1 Enterococci Analysis Using Enterolert IDEXX Method 018 12/19 4 Analytical Balance Calibration 019 2/06 2 pH Procedure 021 12/19 3 Alkalinity Procedure 022 11/16 2 Filtering Water Samples 023 10/18 2 Fecal coliform Analysis Using Colilert 18 IDEXX 024 01/16 2 Method Laboratory Thermometer Calibration 025 04/19 1 Heterotrophic Plate Count – Quanti-tray 026 05/20 1 Appendix A Standard Operating Procedure 001 Date: 11/04 General Laboratory Safety Revision: 1 Author: Linda Green University of Rhode Island Watershed Watch 1.0 PURPOSE AND DESCRIPTION LAB SAFETY IS EVERYBODY’S JOB! Please be sure to familiarize yourself with these general procedures, as well as the specific handling requirements included in the Standard Operating Procedure (SOP) for each analysis/process. Further general information regarding University of Rhode Island standards for health and safety are found in SOP 001a – University Safety and Waste Handling Document. -

Operational Risk Management Guide
OPERATIONAL RISK MANAGEMENT GUIDE U.S. DEPARTMENT OF AGRICULTURE FOREST SERVICE 2020 Last Updated 02/26/2020 RISK MANAGEMENT COUNCIL IN COOPERATION WITH THE OFFICE OF SAFETY & OCCUPATIONAL HEALTH and THE NATIONAL AVIATION SAFETY COUNCIL Contents Contents ....................................................................................................................................................................................... 2 Executive Summary .................................................................................................................................................................. i Introduction ............................................................................................................................................................................... 1 What is Operational Risk Management? ................................................................................................................... 1 The Terminology of ORM ................................................................................................................................................ 1 Principles of ORM Application ........................................................................................................................................... 6 The Five-Step ORM Process ................................................................................................................................................ 7 Step 1: Identify Hazards .................................................................................................................................................. -

Controlling Hazardous Energy: De-Energization and Lockout Iii Trapped-Key Interlock Systems
Controlling Hazardous Energy De-Energization and Lockout About WorkSafeBC At WorkSafeBC, we’re dedicated to promoting safe and healthy workplaces across B.C. We partner with workers and employers to save lives and prevent injury, disease, and disability. When work-related injuries or diseases occur, we provide compensation and support injured workers in their recovery, rehabilitation, and safe return to work. We also provide no-fault insurance and work diligently to sustain our workers’ compensation system for today and future generations. We’re honoured to serve the workers and employers in our province. Prevention Information Line We provide information and assistance with health and safety issues in the workplace. Call the information line 24 hours a day, 7 days a week to report unsafe working conditions, a serious incident, or a major chemical release. Your call can be made anonymously. We can provide assistance in almost any language. If you have questions about workplace health and safety or the Occupational Health and Safety Regulation, call during our office hours (8:05 a.m. to 4:30 p.m.) to speak to a WorkSafeBC officer. If you’re in the Lower Mainland, call 604.276.3100. Elsewhere in Canada, call toll-free at 1.888.621.7233 (621.SAFE). Health and safety resources You can find our health and safety resources on worksafebc.com, and many of them can be ordered from the WorkSafeBC Store at worksafebcstore.com. In addition to books, you’ll find other types of resources at the WorkSafeBC Store, including DVDs, posters, and brochures. If you have any questions about placing an order online, please contact a customer service representative at 604.232.9704 or toll-free at 1.866.319.9704. -

Guide Six Steps to Risk Management
Guide Six step to risk management www.worksafe.nt.gov.au Disclaimer This publication contains information regarding work health and safety. It includes some of your obligations under the Work Health and Safety (National Uniform Legislation) Act – the WHS Act – that NT WorkSafe administers. The information provided is a guide only and must be read in conjunction with the appropriate legislation to ensure you understand and comply with your legal obligations. Acknowledgement This guide is based on material produced by WorkSafe ACT at www.worksafe.act.gov.au Version: 1.2 Publish date: September 2018 Contents Introduction ..............................................................................................................................4 Good management practice.....................................................................................................4 Defining Hazard and Risk.....................................................................................................5 Systematic approach to the management of Hazards and associated Risks ......................5 Step 1: Hazard identification ....................................................................................................6 Examples of Hazards ...........................................................................................................6 Step 2: Risk identification.........................................................................................................7 Examples of risk identification ..............................................................................................7 -
Sampling Guide for First Responders to Drinking Water Contamination Threats and Incidents
Sampling Guide for First Responders to Drinking Water Contamination Threats and Incidents This First Responders Sampling Guide was funded under U.S. EPA Assistance Agreement No. X6-97109101-1. Sampling Guide for First Responders to Drinking Water Contamination Threats and Incidents New England Water Works Association 125 Hopping Brook Road Holliston, MA 01746-1471 November 2008 Acknowledgments This Sampling Guide for First Responders to Drinking Water Contamination Threats and Incidents was originally developed by the New England Water Works Association in collaboration with U.S. EPA. Please refer to pages 75 and 76 for a complete list of acknowledgments. The cover photo is reprinted with permission from the Worcester Telegram & Gazette. IMPORTANT NOTICE The methods and instructions in this guide reflect the U.S. Environmental Protection Agency (EPA) regulations and guidance, in addition to other reference documents. It is recognized that there can be significant differences from state to state regarding public notification, sampling procedures, laboratory safety, and handling, etc. Please check with your state drinking water representative with any questions before First Responders training and preparation is begun. Contents, Sections 1 through 6 1. Introduction 1.1 Background/Purpose ......................................1 1.2 How to Use This Guide ....................................2 2. Overview of Site Characterization .........................3 2.1 Investigating the Site .......................................3 2.2 Who Conducts Site Characterization and Sampling? .................................................5 3. Site Characterization Five-Step Process ...............6 3.1 The Five-Step Process .....................................6 Step 1: Customizing the Site Characterization Plan ........................8 Step 2: Approaching the Site and Doing a Field Safety Screening ........9 Step 3: Characterizing the Site ...................11 Step 4: Collecting Samples ..........................14 Step 5: Exiting the Site .................................16 4. -

Commodity Specific Food Safety Guidelines for the Fresh Tomato Supply Chain
COMMODITY SPECIFIC FOOD SAFETY GUIDELINES FOR THE FRESH TOMATO SUPPLY CHAIN Third Edition | September 2018 Commodity Specific Food Safety Guidelines for the Fresh Tomato Supply Chain Tomato Guidelines, 3rd Edition User’s Note These guidelines provide recommended food safety practices that are intended to minimize the microbiological hazards associated with fresh and fresh-cut tomato products. The intent of drafting this document is to provide currently available information on food safety and handling in a manner consistent with existing applicable regulations, standards and guidelines. The information provided herein is offered in good faith and believed to be reliable, but is made without warranty, express or implied, as to merchantability, fitness for a particular purpose, or any other matter. These recommended guidelines were not designed to apply to any specific operation. It is the responsibility of the user of this document to verify that these guidelines are appropriate for its operation. The publishing trade associations, their members and contributors do not assume any responsibility for compliance with applicable laws and regulations, and recommend that users consult with their own legal and technical advisers to be sure that their own procedures meet with applicable requirements. - 1 - Foreword The North American Tomato Trade Work Group (NATTWG) published in 2006 the first edition of Commodity Specific Food Safety Guidelines for the Fresh Tomato Supply Chain (“Guidelines”). Within two years of publication, several initiatives resulted in significant new learnings about potential risks and control measures at all points in the fresh tomato supply chain. Some of those initiatives include the FDA Tomato Safety Initiative, voluntary efforts by the Florida Tomato Exchange and the California Tomato Farmers to develop USDA-verified audit criteria and programs for tomato production and harvest practices in those states, and several retail and foodservice buyer initiatives to further define tomato safe growing and handling practices. -

Risk Prevention Plan for Contractor Companies. Construction Stage
ANNEX 3 CONTINGENCIES AND RISK PREVENTION MANUAL - Risk Prevention Plan for Contractor Companies. Construction Stage. - Contingency Plan for Contractor Companies. Construction Stage. - Emergency Plan for Cordillera Complex. - Spillage Management Plan for Cordillera Hydro Power Complex. RISK PREVENTION PLAN FOR CONTRACTOR COMPANIES PHAM CONSTRUCTION STAGE May 2008 INTRODUCTION The purpose of this document is to provide the regulatory provisions in Risk Prevention that will rule all contracting activities for works and/or services that AES Gener S.A., hereafter Gener, undertakes with third parties under the umbrella of construction of hydro power houses Alfalfal II and Las Lajas which are part of Alto Maipo Hydro Power Project or PHAM, in order to protect the physical integrity of people rendering services during the execution as well as to prevent risks of accident that compromise Gener's human and material resources. The application of these provisions is mandatory for all and every person involved in the construction works of the Project, either contractors or sub-contractors. In this regard, Gener holds the right to enforce the regulatory provisions stated in the Document herein. It is important to highlight the accuracy of the scopes included in the present document shall be defined and rendered official by Gener once each contractor is awarded the works following the bidding process and taking into account the strategies in terms of risk prevention that each contracted companies has in place. Notwithstanding the above, such provisions shall not be less restrictive than those considered herein. 1. OBJECTIVE Provide provisions and measures that shall rule and guide work contractors and the workers thereof in risk prevention.