Powder Blasted Microsystem for Hatching, Observation and Study of Caridina Multidentata Larvae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Two Freshwater Shrimp Species of the Genus Caridina (Decapoda, Caridea, Atyidae) from Dawanshan Island, Guangdong, China, with the Description of a New Species
A peer-reviewed open-access journal ZooKeys 923: 15–32 (2020) Caridina tetrazona 15 doi: 10.3897/zookeys.923.48593 RESEarcH articLE http://zookeys.pensoft.net Launched to accelerate biodiversity research Two freshwater shrimp species of the genus Caridina (Decapoda, Caridea, Atyidae) from Dawanshan Island, Guangdong, China, with the description of a new species Qing-Hua Chen1, Wen-Jian Chen2, Xiao-Zhuang Zheng2, Zhao-Liang Guo2 1 South China Institute of Environmental Sciences, Ministry of Ecology and Environment, Guangzhou 510520, Guangdong Province, China 2 Department of Animal Science, School of Life Science and Enginee- ring, Foshan University, Foshan 528231, Guangdong Province, China Corresponding author: Zhao-Liang Guo ([email protected]) Academic editor: I.S. Wehrtmann | Received 19 November 2019 | Accepted 7 February 2020 | Published 1 April 2020 http://zoobank.org/138A88CC-DF41-437A-BA1A-CB93E3E36D62 Citation: Chen Q-H, Chen W-J, Zheng X-Z, Guo Z-L (2020) Two freshwater shrimp species of the genus Caridina (Decapoda, Caridea, Atyidae) from Dawanshan Island, Guangdong, China, with the description of a new species. ZooKeys 923: 15–32. https://doi.org/10.3897/zookeys.923.48593 Abstract A faunistic and ecological survey was conducted to document the diversity of freshwater atyid shrimps of Dawanshan Island. Two species of Caridina that occur on this island were documented and discussed. One of these, Caridina tetrazona sp. nov. is described and illustrated as new to science. It can be easily distinguished from its congeners based on a combination of characters, which includes a short rostrum, the shape of the endopod of the male first pleopod, the segmental ratios of antennular peduncle and third maxilliped, the slender scaphocerite, and the absence of a median projection on the posterior margin. -

A Genetical and Ecological Diversity of Fresh Water Prawns Macrobrachium Canarae and Caridina Gracilirostris from Kanyakumari Dist., Tamil Nadu, India
International Journal of Genetic Engineering and Biotechnology. ISSN 0974-3073 Volume 2, Number 1 (2011), pp. 23-32 © International Research Publication House http://www.irphouse.com A Genetical and Ecological Diversity of Fresh Water Prawns Macrobrachium Canarae and Caridina Gracilirostris from Kanyakumari Dist., Tamil Nadu, India Siva Ranjanee S.1 and Mariapan N.2 1Department of Biotechnology, Vels University, Pallavaram, Chennai, India 2Senior Medical Writer, SIRO Clinpharm Pvt. Ltd., Thane (W) 400 607. India E-mail: [email protected], [email protected] Abstract Fresh water prawns are cultured widely around the world but little is known about the levels and patterns of genetic diversity. This paper reports the RAPD analysis of two species of fresh water prawns of Atydiae and Palaemonidiae family and the genus Macrobrachium and Caridina collected from Kanyakumari district. Specimens are identified using species-specific morphological characteristics. Morphological characters have limitations of describing intra specific genetic diversity as they are polygenic and expressions can be modified by the environment. The advent of molecular technique made possible not only the genetic analysis and also the study of evolutionary relationship. Molecular analysis of the morphologically identified species are done by extracting the DNA from the animal and concentration of DNA is measured using Nanodrop spectrophotometer at a wavelength of 280nm. Further analysis was performed with RAPD (Rapid Amplified Polymorphic DNA) a PCR (Polymerase Chain Reaction) based technique. It consist of genomic DNA, amplified with randomly constructed oligonucleotides. Specific quantity of extracted DNA was then amplified by multiplex PCR using random primers and 16S rRNA gene products was then analyzed using a bioanalyzer. -

Evolutionary Origin of Type IV Classical Cadherins in Arthropods Mizuki Sasaki1,4, Yasuko Akiyama-Oda1,2 and Hiroki Oda1,3*
Sasaki et al. BMC Evolutionary Biology (2017) 17:142 DOI 10.1186/s12862-017-0991-2 RESEARCHARTICLE Open Access Evolutionary origin of type IV classical cadherins in arthropods Mizuki Sasaki1,4, Yasuko Akiyama-Oda1,2 and Hiroki Oda1,3* Abstract Background: Classical cadherins are a metazoan-specific family of homophilic cell-cell adhesion molecules that regulate morphogenesis. Type I and type IV cadherins in this family function at adherens junctions in the major epithelial tissues of vertebrates and insects, respectively, but they have distinct, relatively simple domain organizations that are thought to have evolved by independent reductive changes from an ancestral type III cadherin, which is larger than derived paralogs and has a complicated domain organization. Although both type III and type IV cadherins have been identified in hexapods and branchiopods, the process by which the type IV cadherin evolved is still largely unclear. Results: Through an analysis of arthropod genome sequences, we found that the only classical cadherin encoded in chelicerate genomes was the type III cadherin and that the two type III cadherin genes found in the spider Parasteatoda tepidariorum genome exhibited a complex yet ancestral exon-intron organization in arthropods. Genomic and transcriptomic data from branchiopod, copepod, isopod, amphipod, and decapod crustaceans led us to redefine the type IV cadherin category, which we separated into type IVa and type IVb, which displayed a similar domain organization, except type IVb cadherins have a larger number of extracellular cadherin (EC) domainsthandotypeIVacadherins (nine versus seven). We also showed that type IVa cadherin genes occurred in the hexapod, branchiopod, and copepod genomes whereas only type IVb cadherin genes were present in malacostracans.Furthermore,comparative characterization of the type IVb cadherins suggested that the presence of two extra EC domains in their N-terminal regions represented primitive characteristics. -

Caridina Variabilirostris (Crustacea: Decapoda: Atyidae), a New Species
Caridina variabilirostris (Crustacea: Decapoda: Atyidae), a new species of freshwater shrimp from Pohnpei (Micronesia) Valentin de Mazancourt, Gerard Marquet, Philippe Keith To cite this version: Valentin de Mazancourt, Gerard Marquet, Philippe Keith. Caridina variabilirostris (Crustacea: De- capoda: Atyidae), a new species of freshwater shrimp from Pohnpei (Micronesia). European Journal of Taxonomy, Consortium of European Natural History Museums, 2018, pp.1-16. 10.5852/ejt.2018.453. hal-02171169 HAL Id: hal-02171169 https://hal.archives-ouvertes.fr/hal-02171169 Submitted on 2 Jul 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. European Journal of Taxonomy 453: 1–16 ISSN 2118-9773 https://doi.org/10.5852/ejt.2018.453 www.europeanjournaloftaxonomy.eu 2018 · Mazancourt V. de et al. This work is licensed under a Creative Commons Attribution 3.0 License. Research article urn:lsid:zoobank.org:pub:445C8252-5FA2-49AA-AB89-E1C2DDEBEE7F Caridina variabilirostris (Crustacea: Decapoda: Atyidae), a new species of freshwater shrimp from Pohnpei (Micronesia) Valentin de MAZANCOURT 1,*, Gerard MARQUET 2 & Philippe KEITH 3 1,2,3 Muséum national d’Histoire naturelle, Département Adaptations du Vivant, UMR 7208, CP026, 57, rue Cuvier, 75231 Paris, Cedex 05, France. -

Caridina Wild Type Final 1
FRESHWATER SHRIMP WILD TYPE BY CHRIS LUKHAUP Paracaridina meridionalis During the course of evolution, Caridina logemanni They are also the most widely spread Paracaridina meridionalis freshwater dwarf shrimp have spread shrimp in the aquarium hobby. from the estuaries of larger rivers to However, recent research has found rapidly-flowing creeks in the that this genus is in urgent need of mountains,to enormous lakes, the a scientific review and re-structuring smallest trickles of water and even to as there are many discrepancies to cave waters. They are found from be found. The genus Neocaridina tropic to temperate climates today, has up until now been represented however, they have not conquered by 30 species, and has also found wide distribution in the hobby. Paracaridina meridionalis the arctic regions yet. The abundance Paracaridina meridionalis of different habitats has resulted in a Within the Genus Paracaridina just great variability in shrimp species and very few species have been described in stunning forms. Their sometimes but nevertheless some of them are truly impressive colours and patterns found in the shrimpkeeping hobby are the result of their adaptation to the different living conditions in their habitats. Enjoy and keep on shrimping ! With over 290 species, shrimp of the Paracaridina meridionalis genus Caridina are one of the most Paracaridina meridionalis diverse groups within the Atyidae family. Paracaridina meridionalis Paracaridina meridionalis Paracaridina meridionalis Paracaridina meridionalis Paracaridina meridionalis Caridina logemanni Caridina sp. “Vietnam” Caridina serrata Caridina serrata Caridina serrata Caridina trifasciata Caridina trifasciata Caridina trifasciata Caridina trifasciata Caridina sumatrensis paraCaridina sp. Caridina breviata Atyaephyra desmaresti Caridina simoni Caridina gracilirostris caridina serratirostris caridina serratirostris caridina serratirostris caridina serratirostris caridina sp” .Vietnam Blue” Caridina sp. -

L'aquarium: Vision Et Représentation Des Mondes Subaquatiques
L’Aquarium : vision et représentation des mondes subaquatiques : un dispositif d’exposition au croisement de l’art et de la science Quentin Montagne To cite this version: Quentin Montagne. L’Aquarium : vision et représentation des mondes subaquatiques : un dispositif d’exposition au croisement de l’art et de la science. Art et histoire de l’art. Université Rennes 2, 2019. Français. NNT : 2019REN20010. tel-02410780v2 HAL Id: tel-02410780 https://tel.archives-ouvertes.fr/tel-02410780v2 Submitted on 28 Feb 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Thèse soutenue le 07 janvier 2019, devant le jury composé de: L'.Aquarium: Éric Baratay vision et représentation Professeur des universités, Université Jean Moulin Lyon 3 (rapporteur) des mondes subaquatiques Sandrine Ferret Professeure des universités, Université Rennes 2 Un dispositif d'exposition Nicolas Roc'h au croisement de l'art et de la science artiste plasticien Corine Pencenat Maître de conférences HDR, Université de Strasbourg Olivier Schefer Professeur des universités. Université Paris 1 Panthéon-Sorbonne (rapporteur) UNIVERSITE Christophe Viart j:f;jil+iCHII Professeur des unrversités. Université Paris 1 Panthéon-Sorbonne Montagne,l!IJl;J=i Quentin. -
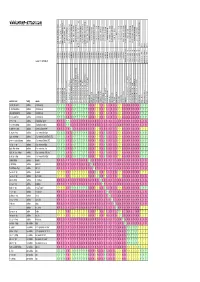
Crossbreeding/Interbreeding Chart Gotten from Web-Site
www.shrimp-attack.com nn blue blue pearl rib bee line ninja shrimp ninja green shrimp orchid shrimp yellow shrimp yellow sakura shrimp tuepfel shrimp orange shrimp yamato shrimp cardinal shrimp red tiger shrimp new new bee shrimp new bee shrimp new new bee shrimp new bee shrimp snowball shrimp snowball goldflake shrimp harlequin shrimp harlequin shrimp blue blue tiger shrimp red cherry shrimp red shrimp hawaii black tiger shrimp crystal red shrimp panda taiwan Bee bumble beebumble shrimp indian zebraindian shrimp crystal black shrimp blue blue bolt taiwan bee red ruby Taiwan bee Towuti/Matano Tiger king king kong Taiwan bee marmoreal dwarfshrimp crystal white bee shrimp golden golden chrystal red shrimp spec. spec. spec. cardina cardina cardina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina caridina neocaridina neocaridina neocaridina neocaridina neocaridina neocaridina version 1.1. / 2010-04-20 halocaridina paracaridina paracaridina paracaridina common name family species cf.cf. cantonensiscantonensis 'Golden 'Golden CRS' CRS' cf.cf. cantonensiscantonensis redred tiger tiger cf.cf. cantonensiscantonensis sp.sp. 'bee' 'bee' cf.cf. cantonensiscantonensis sp.sp. 'white'white bee' bee' cf.cf. cantonensiscantonensis blackblack tiger tiger dennerli glaubrechti holthuisi maculata meridionalis multidentata propinqua serrata 'tuepfel' serratirostris spinata spongicola striata tenuirostris tumida venusta woltereckae rubra cf.cf. var. var.zhangjiajiensis zhangjiajiensis 'blue' 'blue' cf.cf. var. var.zhangjiajiensis zhangjiajiensis 'white' 'white' heteropodaheteropoda var.var. 'red' 'red' heteropodaheteropoda var.var. 'sakura' 'sakura' heteropodaheteropoda var.var. 'yellow' 'yellow' palmata blue bee princess bee larry shrimp cantonensis sp. cantonensis sp. -

First Comprehensive Analysis of Lysine Acetylation in Alvinocaris Longirostris from the Deep-Sea Hydrothermal Vents Min Hui1,2, Jiao Cheng1,2 and Zhongli Sha1,2,3*
Hui et al. BMC Genomics (2018) 19:352 https://doi.org/10.1186/s12864-018-4745-3 RESEARCH ARTICLE Open Access First comprehensive analysis of lysine acetylation in Alvinocaris longirostris from the deep-sea hydrothermal vents Min Hui1,2, Jiao Cheng1,2 and Zhongli Sha1,2,3* Abstract Background: Deep-sea hydrothermal vents are unique chemoautotrophic ecosystems with harsh conditions. Alvinocaris longirostris is one of the dominant crustacean species inhabiting in these extreme environments. It is significant to clarify mechanisms in their adaptation to the vents. Lysine acetylation has been known to play critical roles in the regulation of many cellular processes. However, its function in A. longirostris and even marine invertebrates remains elusive. Our study is the first, to our knowledge, to comprehensively investigate lysine acetylome in A. longirostris. Results: In total, 501 unique acetylation sites from 206 proteins were identified by combination of affinity enrichment and high-sensitive-massspectrometer. It was revealed that Arg, His and Lys occurred most frequently at the + 1 position downstream of the acetylation sites, which were all alkaline amino acids and positively charged. Functional analysis revealed that the protein acetylation was involved in diverse cellular processes, such as biosynthesis of amino acids, citrate cycle, fatty acid degradation and oxidative phosphorylation. Acetylated proteins were found enriched in mitochondrion and peroxisome, and many stress response related proteins were also discovered to be acetylated, like arginine kinases, heat shock protein 70, and hemocyanins. In the two hemocyanins, nine acetylation sites were identified, among which one acetylation site was unique in A. longirostris when compared with other shallow water shrimps. -

Assessment of the Risk to Norwegian Biodiversity from Import and Keeping of Crustaceans in Freshwater Aquaria
VKM Report 2021: 02 Assessment of the risk to Norwegian biodiversity from import and keeping of crustaceans in freshwater aquaria Scientific Opinion of the Panel on Alien Organisms and Trade in Endangered Species of the Norwegian Scientific Committee for Food and Environment VKM Report 2021: 02 Assessment of the risk to Norwegian biodiversity from import and keeping of crustaceans in freshwater aquaria. Scientific Opinion of the Panel on Alien Organisms and trade in Endangered Species (CITES) of the Norwegian Scientific Committee for Food and Environment 15.02.2021 ISBN: 978-82-8259-356-4 ISSN: 2535-4019 Norwegian Scientific Committee for Food and Environment (VKM) Postboks 222 Skøyen 0213 Oslo Norway Phone: +47 21 62 28 00 Email: [email protected] vkm.no vkm.no/english Cover photo: Mohammed Anwarul Kabir Choudhury/Mostphotos.com Suggested citation: VKM, Gaute Velle, Lennart Edsman, Charlotte Evangelista, Stein Ivar Johnsen, Martin Malmstrøm, Trude Vrålstad, Hugo de Boer, Katrine Eldegard, Kjetil Hindar, Lars Robert Hole, Johanna Järnegren, Kyrre Kausrud, Inger Måren, Erlend B. Nilsen, Eli Rueness, Eva B. Thorstad and Anders Nielsen (2021). Assessment of the risk to Norwegian biodiversity from import and keeping of crustaceans in freshwater aquaria. Scientific Opinion of the Panel on Alien Organisms and trade in Endangered Species (CITES) of the Norwegian Scientific Committee for Food and Environment. VKM report 2021:02, ISBN: 978-82-8259- 356-4, ISSN: 2535-4019. Norwegian Scientific Committee for Food and Environment (VKM), Oslo, Norway. 2 Assessment of the risk to Norwegian biodiversity from import and keeping of crustaceans in freshwater aquaria Preparation of the opinion The Norwegian Scientific Committee for Food and Environment (Vitenskapskomiteen for mat og miljø, VKM) appointed a project group to draft the opinion. -

Lines of Evidence for the Validity of Caridina Buehleri Roux (Crustacea : Decapoda : Atyidae) and for C
When molecules and morphology work together: lines of evidence for the validity of Caridina buehleri Roux (Crustacea : Decapoda : Atyidae) and for C. gueryi Marquet, Keith & Kalfatak as its junior synonym Valentin de Mazancourt, Gerard Marquet, Werner Klotz, Philippe Keith, Magalie Castelin To cite this version: Valentin de Mazancourt, Gerard Marquet, Werner Klotz, Philippe Keith, Magalie Castelin. When molecules and morphology work together: lines of evidence for the validity of Caridina buehleri Roux (Crustacea : Decapoda : Atyidae) and for C. gueryi Marquet, Keith & Kalfatak as its junior synonym. Invertebrate Systematics, CSIRO Publishing, 2017, 31 (2), pp.220. 10.1071/IS16044. hal-02171153 HAL Id: hal-02171153 https://hal.archives-ouvertes.fr/hal-02171153 Submitted on 2 Jul 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 1 When molecules and morphology work together: lines of evidence for the validity of 2 Caridina buehleri Roux, 1934 (Crustacea: Decapoda: Atyidae) and for C. gueryi Marquet, 3 Keith & Kalfatak, 2009 as its junior synonym. 4 VALENTIN de MAZANCOURT1*, GERARD MARQUET1, WERNER KLOTZ3, PHILIPPE 5 KEITH1 AND MAGALIE CASTELIN1-2 6 7 1 Muséum national d’Histoire naturelle, DMPA, UMR 7208, CP026, 57, rue Cuvier, F-75231 8 Paris, Cedex 05 9 2 Aquatic Animal Health Section, Fisheries and Oceans Canada, Pacific Biological Station, 10 3190 Hammond Bay Road, Nanaimo, BC, Canada V9T 6N7 11 3 Wiesenweg 1, A-6063 Rum, Austria. -

FOUR NEW PHILIPPINE SPECIES of FRESH-WATER the Description Of
Philippine Journal of Science, vol. 70, Bo. k December, 1939 D FOUR NEW PHILIPPINE SPECIES OF FRESH-WATER Ui SHRIMPS OF THE GENUS CARIDINA Q £ By GUILLERMO J. BLANCO Of the Division of Fisheries, Department of Agriculture and Commerce Manila THREE PLATES The description of these apparently new species of shrimps is based upon material collected from Laoag River, Laoag, Ilocos Norte Province, December 31, 1938, by Mr. Eulogio J. Martinez, and from a mountain stream, Helosig, Leyte, 1,500 feet above sea level, May 23, 1937, by Messrs. Dioscoro S. Rabor and M. Celestino, both of the Division of Fisheries, Bureau of Science. Genus CARIDINA Milne-Edwards Caridina MILNE-EDWARDS, Histoire Naturelle des Crustaces 2 (1837). C ARID IN A VILLADOLIDI sp. nov. Plate 1, figs. 1 to 9. Rostrum straight, saberlike, nearly reaching level of antennal scale; upper edge without teeth; lower edge with 6 teeth on distal half of its total length; a pair of setae posterior of rostrum (Plate 1, fig. 1). Antennal spine sharp; anteroinferior angle of carapace sharp-pointed. Eyes normal. Antennular pedun- cle reaching beyond tip of spine of antennular peduncle scale; basal segment not reaching beyond half of length of rostrum; second segment long, twice its width. Antennal scale slender, three times as long as broad. Mandible with three spines of incisor process and setse (Plate 1, fig. 2). Terminal joint of third maxilliped (Plate 1, fig. 3) with eight spines and nu- merous setse. Carpus of first perseopod (Plate 1, fig. 4) twice as long as distal breadth, excavation at distal end slightly cres- cent-shaped. -

A Morphological and Molecular Study of Diversity and Connectivity Among Anchialine Shrimp
Florida International University FIU Digital Commons FIU Electronic Theses and Dissertations University Graduate School 11-10-2020 Connections in the Underworld: A Morphological and Molecular Study of Diversity and Connectivity among Anchialine Shrimp. Robert Eugene Ditter Florida International University, [email protected] Follow this and additional works at: https://digitalcommons.fiu.edu/etd Part of the Biodiversity Commons, Bioinformatics Commons, Biology Commons, Ecology and Evolutionary Biology Commons, Genetics and Genomics Commons, Marine Biology Commons, Natural Resources and Conservation Commons, Oceanography Commons, Other Environmental Sciences Commons, Speleology Commons, and the Zoology Commons Recommended Citation Ditter, Robert Eugene, "Connections in the Underworld: A Morphological and Molecular Study of Diversity and Connectivity among Anchialine Shrimp." (2020). FIU Electronic Theses and Dissertations. 4561. https://digitalcommons.fiu.edu/etd/4561 This work is brought to you for free and open access by the University Graduate School at FIU Digital Commons. It has been accepted for inclusion in FIU Electronic Theses and Dissertations by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. FLORIDA INTERNATIONAL UNIVERSITY Miami, Florida CONNECTIONS IN THE UNDERWORLD: A MORPHOLOGICAL AND MOLECULAR STUDY OF DIVERSITY AND CONNECTIVITY AMONG ANCHIALINE SHRIMP A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in BIOLOGY by Robert E. Ditter 2020 To: Dean Michael R. Heithaus College of Arts, Sciences and Education This dissertation, written by Robert E. Ditter, and entitled Connections in the Underworld: A Morphological and Molecular Study of Diversity and Connectivity among Anchialine Shrimp, having been approved in respect to style and intellectual content, is referred to you for judgment.