Kaolinite from Warsaw Geodes, Keokuk Region, Iowa
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download PDF About Minerals Sorted by Mineral Name
MINERALS SORTED BY NAME Here is an alphabetical list of minerals discussed on this site. More information on and photographs of these minerals in Kentucky is available in the book “Rocks and Minerals of Kentucky” (Anderson, 1994). APATITE Crystal system: hexagonal. Fracture: conchoidal. Color: red, brown, white. Hardness: 5.0. Luster: opaque or semitransparent. Specific gravity: 3.1. Apatite, also called cellophane, occurs in peridotites in eastern and western Kentucky. A microcrystalline variety of collophane found in northern Woodford County is dark reddish brown, porous, and occurs in phosphatic beds, lenses, and nodules in the Tanglewood Member of the Lexington Limestone. Some fossils in the Tanglewood Member are coated with phosphate. Beds are generally very thin, but occasionally several feet thick. The Woodford County phosphate beds were mined during the early 1900s near Wallace, Ky. BARITE Crystal system: orthorhombic. Cleavage: often in groups of platy or tabular crystals. Color: usually white, but may be light shades of blue, brown, yellow, or red. Hardness: 3.0 to 3.5. Streak: white. Luster: vitreous to pearly. Specific gravity: 4.5. Tenacity: brittle. Uses: in heavy muds in oil-well drilling, to increase brilliance in the glass-making industry, as filler for paper, cosmetics, textiles, linoleum, rubber goods, paints. Barite generally occurs in a white massive variety (often appearing earthy when weathered), although some clear to bluish, bladed barite crystals have been observed in several vein deposits in central Kentucky, and commonly occurs as a solid solution series with celestite where barium and strontium can substitute for each other. Various nodular zones have been observed in Silurian–Devonian rocks in east-central Kentucky. -

Fire Retardancy of Polypropylene/Kaolinite Composites Marcos Batistella, Belkacem Otazaghine, Rodolphe Sonnier, Carlos Petter, José-Marie Lopez-Cuesta
Fire retardancy of polypropylene/kaolinite composites Marcos Batistella, Belkacem Otazaghine, Rodolphe Sonnier, Carlos Petter, José-Marie Lopez-Cuesta To cite this version: Marcos Batistella, Belkacem Otazaghine, Rodolphe Sonnier, Carlos Petter, José-Marie Lopez-Cuesta. Fire retardancy of polypropylene/kaolinite composites. Polymer Degradation and Stability, Elsevier, 2016, 129, pp.260-267. 10.1016/j.polymdegradstab.2016.05.003. hal-02906432 HAL Id: hal-02906432 https://hal.archives-ouvertes.fr/hal-02906432 Submitted on 26 May 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Fire retardancy of polypropylene/kaolinite composites * Marcos Batistella a, c, , Belkacem Otazaghine b, Rodolphe Sonnier b, Carlos Petter c, Jose-Marie Lopez-Cuesta b a Federal University of Santa Catarina, R. Eng. Agronomico^ Andrei Cristian Ferreira, s/n e Trindade, Florianopolis, SC, CEP 88040-900, Brazil b Ecole des Mines d’Ales, Centre des Materiaux (C2MA) e Pole^ Materiaux Polymeres Avances, 6 Avenue de Clavieres, 30319, Ales Cedex, France c Federal University of Rio Grande do Sul, Av. Bento Gonçalves, 9500, Porto Alegre, CEP 91501-970, Brazil abstract In this study the influence of surface modification of kaolinite with trisilanolisooctyl Polyhedral Oligo- SilSesquioxane (POSS) in polypropylene composites was evaluated in terms of thermal stability and fire retardancy and compared with talc. -

Evaluation of Selected Kaolin Clays As a Raw Material for the Turkish Cement and Concrete Industry
Evaluation of Selected Kaolin Clays as a Raw Material for the Turkish Cement and Concrete Industry Aydin Aras1, Mustafa Albayrak1, Metin Arikan2, Konstantin Sobolev3 * 1 General Directorate of Mineral Research and Exploration (MTA), Turkey 2 Civil Engineering Department, Middle East Technical University (METU), Turkey 3 Facultad de Ingenieria Civil, Universidad Autonoma de Nuevo Leon (UANL), Mexico ABSTRACT Turkey has a long tradition (starting from the prehistoric civilizations) and experience in exploring and processing clay raw materials into ceramic products. Many of these products, such as tiles and sanitary ware, are manufactured for domestic and export markets. Kaolin clay is one of the raw materials of major importance for the ceramic and paper industry, as well as for a number of auxiliary applications. There is an ongoing interest to apply kaolin clay in the construction industry as a raw material for the production of white cement clinker and as an artificial pozzolanic additive for concrete (in a form of metakaolin). This report presents the results related to search, assessment and evaluation of available resources for advanced cement and concrete additives. Keywords: kaolin, metakaolin, construction, resources, ceramics, cement, x-ray diffraction, SEM INTRODUCTION Turkey has an abundance of natural resources and its mining industry is one of the sectors showing steady growth. Among the most commonly mined minerals are borax, magnesite, chromites, barite, feldspars, different clays, and limestone [1-21]. Local ceramic industry has more than 4000 years of experience in exploring and processing widely available raw materials into useful commodities. Currently, several ceramic products, such as tiles and sanitary ware are manufactured to meet international standards (ISO 9000) and significant amounts (about 45 %) of these products are exported [21]. -

Clay Minerals Soils to Engineering Technology to Cat Litter
Clay Minerals Soils to Engineering Technology to Cat Litter USC Mineralogy Geol 215a (Anderson) Clay Minerals Clay minerals likely are the most utilized minerals … not just as the soils that grow plants for foods and garment, but a great range of applications, including oil absorbants, iron casting, animal feeds, pottery, china, pharmaceuticals, drilling fluids, waste water treatment, food preparation, paint, and … yes, cat litter! Bentonite workings, WY Clay Minerals There are three main groups of clay minerals: Kaolinite - also includes dickite and nacrite; formed by the decomposition of orthoclase feldspar (e.g. in granite); kaolin is the principal constituent in china clay. Illite - also includes glauconite (a green clay sand) and are the commonest clay minerals; formed by the decomposition of some micas and feldspars; predominant in marine clays and shales. Smectites or montmorillonites - also includes bentonite and vermiculite; formed by the alteration of mafic igneous rocks rich in Ca and Mg; weak linkage by cations (e.g. Na+, Ca++) results in high swelling/shrinking potential Clay Minerals are Phyllosilicates All have layers of Si tetrahedra SEM view of clay and layers of Al, Fe, Mg octahedra, similar to gibbsite or brucite Clay Minerals The kaolinite clays are 1:1 phyllosilicates The montmorillonite and illite clays are 2:1 phyllosilicates 1:1 and 2:1 Clay Minerals Marine Clays Clays mostly form on land but are often transported to the oceans, covering vast regions. Kaolinite Al2Si2O5(OH)2 Kaolinite clays have long been used in the ceramic industry, especially in fine porcelains, because they can be easily molded, have a fine texture, and are white when fired. -

Kaolinite Al2si2o5(OH)4 C 2001 Mineral Data Publishing, Version 1.2 ° Crystal Data: Triclinic
Kaolinite Al2Si2O5(OH)4 c 2001 Mineral Data Publishing, version 1.2 ° Crystal Data: Triclinic. Point Group: 1: Rarely as crystals, thin platy or stacked, to 2 mm. More commonly as microscopic pseudohexagonal plates and clusters of plates, aggregated into compact, claylike masses. Physical Properties: Cleavage: Perfect on 001 . Tenacity: Flexible but inelastic. Hardness = 2{2.5 D(meas.) = 2.61{2.68 D(caflc.) =g 2.63 Optical Properties: Transparent to translucent as single crystals. Color: White to tan, may be variously colored by impurities. Luster: Pearly to dull earthy. Optical Class: Biaxial ({). Orientation: X c = 13± to 10±; Y a = 1±{4±. Dispersion: r > v; weak. ® = 1.553{1.565^ ¯ =¡1.559{1¡.569 ° =^ 1.560{1.570 2V(meas.) = 24±{50± Cell Data: Space Group: P 1: a = 5.15 b = 8.95 c = 7.39 ® = 91:8± ¯ = 104:5± 105:0± ° = 90± Z = [2] ¡ X-ray Powder Pattern: Scalby, Yorkshire, England (1A). 7.16 (vvs), 3.573 (vvs), 4.336 (vs), 2.491 (s), 2.289 (s), 2.558 (ms), 2.379 (ms) Chemistry: (1) SiO2 45.80 Al2O3 39.55 Fe2O3 0.57 FeO 0.18 MgO 0.14 CaO 0.41 K2O 0.03 + H2O 13.92 H2O¡ 0.17 Total 100.77 3+ (1) Mikawo mine, Niigata Prefecture, Japan; corresponds to (Al2:00Fe0:02Mg0:01Ca0:02)§=2:05 Si2O5(OH)3:99: Polymorphism & Series: Dickite, halloysite, and nacrite are polymorphs. Mineral Group: Kaolinite-serpentine group. Occurrence: Replaces other aluminosilicate minerals during hydrothermal alteration and weathering. A common constituent of the clay-size fraction of sediments, where it may be formed by direct precipitation. -

The Influence of Halloysite Content on the Shear Strength of Kaolinite
Portland State University PDXScholar Dissertations and Theses Dissertations and Theses 1981 The influence of halloysite content on the shear strength of kaolinite Reka Katalin Gabor Portland State University Follow this and additional works at: https://pdxscholar.library.pdx.edu/open_access_etds Part of the Geology Commons, and the Materials Science and Engineering Commons Let us know how access to this document benefits ou.y Recommended Citation Gabor, Reka Katalin, "The influence of halloysite content on the shear strength of kaolinite" (1981). Dissertations and Theses. Paper 3215. https://doi.org/10.15760/etd.3206 This Thesis is brought to you for free and open access. It has been accepted for inclusion in Dissertations and Theses by an authorized administrator of PDXScholar. Please contact us if we can make this document more accessible: [email protected]. AN ABSTRACT OF THE THESIS OF Reka Katalin Gabor for the Master of Science in Geology presented October 6, 1981. Title: The Influence of Halloysite Content on the Shear Strength of Kaolinite. APPROVED BY MEMBERS OF THE THESIS COMMITTEE: The objective of this thesis is to determine the rel ative shear strengths of halloysite, kaolinite, synthetic mixtures, and local soils, to investigate the influence of halloysite content on the shear strength of kaolinite, and to explore the possibility that the strength properties of soil clays might be controlled by the relative content of their component minerals. Sets of samples of pure kaolinite and halloysite min erals and their mixtures in proportions of 1:1, 3:1, and 2 1:3 were prepared in the Harvard Miniature Compaction de vice, each compacted in four separate layers with 35 tamp- ings from the 30 pound spring compactor on each layer. -

Project Geode
Illinois State Museum Geology Online – http://geologyonline.museum.state.il.us Project Geode Grade Level: 7-8 Purpose: To predict the appearance of a geode’s internal structure based on its physical characteristics, such as mass and density. Suggested Goals: Students will collect accurate data about the physical characteristics of a geode and determine a method for predicting the internal structure. Objectives: As a result of this lesson, students will be able to: 1. Use scientific equipment to collect data related to mass, volume, and density. 2. Form a hypothesis about a geode’s interior structures based on collected data. 3. Identify the common minerals found in Illinois geodes. Time Required: 1 - 2 class periods Group Size: Individually or by working in teams of 2-3 students Background: Geodes are spherical bodies that may be filled with layers of minerals and/or lined with crystals, such as quartz, calcite, pyrite, and dolomite. Geodes form as mineral-rich water is trapped inside an outer core of solid rock, including a layer of chalcedony. As the temperature and pressure changes, the mineral matter inside the geode precipitates to form a crystal lining. Some geodes are filled completely, while others are hollow with a beautiful display of crystals. Does the geode provide clues as to its internal structure? For this experiment, challenge your students to document the physical characteristics of a geode and make a prediction as to the internal appearance of a geode. Materials/Preparation: Set of geodes for display (already cracked open to show crystal structures) 8-10 unbroken geodes* (Labeled using 1,2,3, etc.) Triple-beam balances or electronic scales Overflow cans and graduated cylinders (or other method to measure volume) Measuring tapes Safety goggles Hammer & Chisel Optional: Classroom display with minerals that are often found inside geodes, such as quartz, dolomite, calcite, etc. -
Geodes: a Look at Iowa's State Rock
Iowa Science Teachers Journal Volume 26 Number 1 Article 6 1989 Geodes: A Look at Iowa's State Rock Brian J. Witzke Follow this and additional works at: https://scholarworks.uni.edu/istj Part of the Science and Mathematics Education Commons Let us know how access to this document benefits ouy Copyright © Copyright 1989 by the Iowa Academy of Science Recommended Citation Witzke, Brian J. (1989) "Geodes: A Look at Iowa's State Rock," Iowa Science Teachers Journal: Vol. 26 : No. 1 , Article 6. Available at: https://scholarworks.uni.edu/istj/vol26/iss1/6 This Article is brought to you for free and open access by the Iowa Academy of Science at UNI ScholarWorks. It has been accepted for inclusion in Iowa Science Teachers Journal by an authorized editor of UNI ScholarWorks. For more information, please contact [email protected]. GEODES: A LOOK AT IOWA'S STATE ROCK Brian J. Witzke Research Geologist 123 North Capitol Street Iowa City, Iowa 52242 Iowa geodes have long been objects of curiosity, their sparkling interiors containing some of the most beautiful crystals to be found anywhere in the Midwest. Although geodes are known from many localities around the world, one of the most productive and famous collecting regions is encompassed within a 35-mile radius of Keokuk, Iowa. Rock collectors commonly refer to geodes from this region as "Keokuk geodes." In keeping with the world-renowned status of the Iowa geodes, the Iowa General Assembly declared the geode as the official "State Rock" in 1967. The word "geode" is derived from the Latin meaning "earthlike," a reference to their rounded shape. -

Cool Crystals! April 30 Virtual STEAM Club
Cool Crystals! April 30 Virtual STEAM Club Geodes are sphere-shaped rock formations that look very plain and ordinary on the outside, but if you break into their shells, you find that they are often hollow and lined with beautiful crystals and colors. Crystals have many flat sides to reflect light and that gives them their sparkle. The most common crystal you can find on the inside of a geode is quartz. Geodes are abundant in the Midwest (including Southern Indiana!) because they erode from limestone bedrock and roll downstream. They can often be found scattered along creeks and beaches. In sedimentary rock, geodes form inside hollows or cavities, like those made by a tree root, animal, or a mud ball. Around this hollow space, a shell forms and hardens. If water containing tiny pieces of minerals slowly fills up the space beneath the shell, then crystals can form inside. It can take millions of years for the crystals to grow and the space to fill up. Most geodes are not entirely filled with crystals, but if they are completely solid, then they are called nodules. Geodes can also be found in volcanic rock, where the crystals form inside gas bubbles. Today, make a cluster of crystals that will resemble the inside of a geode! Materials: ● 3 Tablespoons Borax ● Dish cloth ● 1 Cup boiling water ● Skewer, butter knife, ● Food coloring or chopstick ● Pipe cleaners ● String or thread ● Large jar, glass or pitcher (3+ cup capacity) Steps: 1. Heat two cups of water to boiling. 2. While your water is heating, take three pipe cleaners and intertwine them into a loose ball. -

Effect of Kaolinite and Ca-Montmorillonite on the Alleviation of Soil Water Repellency
Effect of kaolinite and Ca-montmorillonite on the alleviation of soil water repellency P. Dlapa1, S.H. Doerr2, Ľ. Lichner3, M. Šír4, M. Tesař4 1Faculty of Natural Science, Comenius University, Bratislava, Slovakia 2Department of Geography, University of Wales Swansea, Swansea, UK 3Institute of Hydrology, Slovak Academy of Sciences, Bratislava, Slovakia 4Institute for Hydrodynamics, Academy of Sciences of the Czech Republic, Prague, Czech Republic ABSTRACT The effects of adding 1–3% (weight) kaolinite or Ca-montmorillonite on the we�ability of silica sand, made highly water repellent with stearic acid, was studied during we�ing and prolonged drying phases at 50°C. The persistence of water repellency was estimated with the water drop penetration time (WDPT) test. A�er we�ing water repellency disappeared in all the samples. During the drying phase, water repellency re-appeared in all samples (untreated and clay-treated) as the water content decreased below 1%. Repellency did, however, not reach pre-we�ing levels. The effect of clay additions on water repellency differed strongly between the two clay types. Kaolinite reduced WDPT, while Ca-montmorillonite caused an increase in WDPT in the already highly repellent sand. Potential mechanisms for the alleviation effectiveness of kaolinite are proposed, with key factors being the high adhesion forces between water and clay mineral surfaces, and the ability kaolinite to disperse. In the case of Ca-montmorillonite, its lower affinity for water may lead to a displacement of water molecules at mineral surfaces by amphiphilic organic compounds, which may result in increased repellency. This phenomenon clearly requires further investigation. Keywords: water repellency; kaolinite; Ca-montmorillonite; stearic acid The occurrence of water repellency in many soils to wind erosion. -

Gem, Mineral, Fossil & Jewelry Dealers
Gem, Mineral, Jewelry, & Fossil Vendors Accent on Nature Columbus, OH Amber America Bath, PA Cincinnati Arrowwood Minerals Lexington, VA Aurora Mineral Corp. (wholesale) Freeport, NY Bernie’s Gems Seneca, SC © Butterflies by God Maryland Heights, MO GeoFair 2019 Carved Opal and Obsidian Austin, TX Cecilia Gems with a Smile Columbus, OH 54th Annual Chuck Warren Fossils Hillsdale, MI The Crystal Circle Morrow, OH Gem, Mineral, Fossil & Jewelry Designs Mining & Lapidary Belle Haven, VA Show of Greater Cincinnati Dwarven Stonecraft & Lapidary Nashville, TN Exotic Minerals of Russia Princeton Junction, NJ www.geofair.com Galaxy Marketing Douglasville, GA Gem Miracles Maineville, OH Gems by Celestial Dancer Carmel, IN May 4 Gems of the Ozarks Walnut Ridge, AR Saturday GeoRarities.com Cincinnati, OH 10 am to 6 pm Heads Up Trading Co. Cincinnati, OH Heart Vision Canton, MI May 5 Hollow Mountain Industries Louisville, KY Sunday Howard Schlansker (wholesale) Marshfield, MA 11 am to 5 pm Import Specialist West Chester, OH Ken and Pam Samulski South Bend, IN Kentucky Rock Shop Crittenden, KY Larimar International Amboy, IL 70 Displays featuring Mannings Rock Shop Troy, OH Mearth Star Sunman, IN Colorful Crystals & Midwest Minerals (wholesale) Tucson, AZ Fascinating Fossils Minerals Plus Dowagiac, MI Mountain Minerals International Louisville, CO Natural Selection Crystals Milwaukee, WI Illustrated Earth Science Programs North Lake Trading Avon Lake, OH Saturday, May 4 One of a Kind Jewelry Designer Cabs Branson, MO The Causes of Color in Crystals PhatRocks Knoxville, TN Pheasant Run East Troy, WI The Architecture of Echinoderms The Prospector’s Shop Ligonier, PA Sunday, May 5 Quest Crystals Warren, OH A Rainbow of Gems & Minerals R&D Xtals Cleveland, OH Riverview Gems and Gifts Bandon, OR Building a Dinosaur Rock Candy Minerals Springfield, IL 9743 Roy Hurlburt Minerals St. -
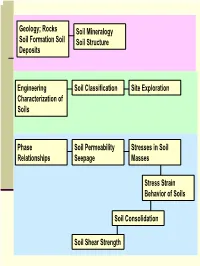
Rocks Soil Formation Soil Deposits Soil Mineralogy Soil Structure
Geology; Rocks Soil Mineralogy Soil Formation Soil Soil Structure Deposits Engineering Soil Classification Site Exploration Characterization of Soils Phase Soil Permeability Stresses in Soil Relationships Seepage Masses Stress Strain Behavior of Soils Soil Consolidation Soil Shear Strength 1 Engineering Characterization of Soils Soil Properties that Control its Engineering Behavior Particle Size − Sieve Analysis − Hydrometer Analysis coarse-grained fine-grained Particle/Grain Size Soil Plasticity Distribution Particle Shapes (?) 2 Particle Size; Standard Sieve Sizes 3 ASTM Particle Size Classification 4 Sieve Analysis (Mechanical Analysis) This procedure is suitable for coarse grained soils See next slide for ASTM Standard Sieves No.10 sieve …. Has 10 apertures per linear inch 5 ASTM Standard Sieves 6 Hydrometer Analysis Also called Sedimentation Analysis Stoke’s Law D2γ (G − G ) v = w s L 18η 7 Grain Size Distribution Curves 8 Terminology C….. Poorly-graded soil D …. Well-graded soil E …. Gap-graded soil D10, D30, D60 = ?? Coefficient of Uniformity, Cu= D60/D10 Coefficient of Curvature, )2 Cc= (D30 /(D10)(D60) 9 Particle Distribution Calculations Example 10 Particle Shapes 11 Clay Formation Clay particles < 2 µm Compared to Sands and Silts, clay size particles have undergone a lot more “chemical weathering”! 12 Clay vs. Sand/Silt Clay particles are generally more platy in shape (sand more equi-dimensional) Clay particles carry surface charge Amount of surface charge depends on type of clay minerals Surface charges that