Gut Microbiota About Rotten-Skin Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Zoosystema 28(3)
Phylogenetic relationships and generic taxonomy of the tribe Paini (Amphibia, Anura, Ranidae, Dicroglossinae), with diagnoses of two new genera Annemarie OHLER Alain DUBOIS Muséum national d’Histoire naturelle, Département Systématique et Évolution, Reptiles et Amphibiens, USM 0602, case postale 30, 25 rue Cuvier, F-75231 Paris cedex 05 (France) [email protected] Ohler A. & Dubois A. 2006. — Phylogenetic relationships and generic taxonomy of the tribe Paini (Amphibia, Anura, Ranidae, Dicroglossinae), with diagnoses of two new genera. Zoosystema 28 (3) : 769-784. ABSTRACT KEY WORDS A preliminary cladistic analysis of the relationships between 26 frog species Amphibia, of the tribe Paini (Ranidae, Dicroglossinae) was carried out on the basis of 31 Anura, Ranidae, morphological characters, mainly from external morphology of adults. Combined Dicroglossinae, with the results of a molecular analysis published elsewhere, these data 1) confi rm Paini, that, after exclusion of the species Rana delacouri Angel, 1928, the Paini are a morphology, cladistic relationships, homophyletic group, and 2) allow to redefi ne the genera of this tribe, which new genera. are now six in number, including two new ones described herein. RÉSUMÉ Relations phylogénétiques et taxonomie générique de la tribu Paini (Amphibia, Anura, Ranidae, Dicroglossinae), avec diagnoses de deux nouveaux genres. Une analyse cladistique préliminaire des relations entre 26 espèces de grenouilles MOTS CLÉS de la tribu des Paini (Ranidae, Dicroglossinae) a été menée en utilisant 31 Amphibia, caractères morphologiques, principalement de la morphologie externe des Anura, Ranidae, adultes. Combinées avec les résultats d’une analyse moléculaire publiée ailleurs, Dicroglossinae, ces données 1) confi rment qu’après exclusion de l’espèce Rana delacouri Angel, Paini, 1928, les Paini constituent un groupe homophylétique, et 2) permettent de morphologie, analyse cladistique, redéfi nir les genres de cette tribu, qui sont maintenant au nombre de six, dont genres nouveaux. -

Progress and Prospects for Studies on Chinese Amphibians
Asian Herpetological Research 2010, 1(2): 64-85 DOI: 10.3724/SP.J.1245.2010.00064 Progress and Prospects for Studies on Chinese Amphibians FEI Liang, YE Changyuan and JIANG Jianping* Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, Sichuan, China Abstract This work summarizes the history and progress of the studies on Chinese amphibians since they first ap- peared in the Chinese literature. A wide range of research has been carried out, including the history of the definition of amphibians, faunal surveys, systematic research, ecological research, biochemical research (isozyme and other proteins or peptides, chromosomes, DNA), anatomical research, embryological research, phylogenetic and zoogeographical re- search, and many others such as ultrastructure of organs, crossbreeding test, regeneration of organs, abnormality survey, acoustics, fossils, sperm ultrastructure and parasites. In addition, the prospects for studies on Chinese amphibians in future are proposed in this paper. Keywords progress, prospect, faunal survey, systematics, amphibian, China 1. Introduction on amphibian research for at least 3000 years: toads (maybe Bufo gargarizans) were equated to ugliness and China is located in east Asia and covers a land area of wickedness in ‘The Book of Songs’ (-3000 years ago), approximately 9.6 million km2. Due to the vast territory but frog (鼃, 黽) had been inscribed on bones or tortoise occupied by this country, extremely different landforms, shell approximately 16th − 11th centuray B.C. (Guo et al., complex environments, and diverse climates and vegeta- 1999). “人鱼” (Mermen, now called the Chinese giant tion, China is very rich in amphibians, not only contai- salamander, Andrias davidianus), “活师” ( meaning tad- ning extremely numerous rare and endemic species, but poles), and “黾” (meaning frogs) were mentioned in ‘The also preserving a large number of relic species. -
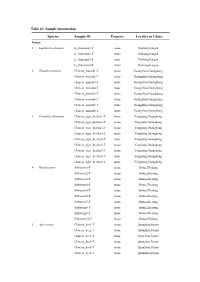
Table S1. Sample Information. Species Sample ID
Table S1. Sample information. Species Sample ID Property Locality in China Insects 1 Luehdorfia chinensis L_chinensis1-T tissue Nanking,Jiangsu L_chinensis2-T tissue Nanking,Jiangsu L_chinensis3-T tissue Nanking,Jiangsu L_chinensis4-T tissue Nanking,Jiangsu 2 Tenodera sinensis Chinese_mantid1-T tissue Guangzhou,Guangdong Chinese_mantid2-T tissue Guangzhou,Guangdong Chinese_mantid3-T tissue Guangzhou,Guangdong Chinese_mantid4-T tissue Guangzhou,Guangdong Chinese_mantid5-T tissue Guangzhou,Guangdong Chinese_mantid6-T tissue Guangzhou,Guangdong Chinese_mantid7-T tissue Guangzhou,Guangdong Chinese_mantid8-T tissue Guangzhou,Guangdong 3 Cicindela chinenesis Chinese_tiger_beetles1-T tissue Yangjiang,Guangdong Chinese_tiger_beetles2-T tissue Yangjiang,Guangdong Chinese_tiger_beetles3-T tissue Yangjiang,Guangdong Chinese_tiger_beetles4-T tissue Yangjiang,Guangdong Chinese_tiger_beetles5-T tissue Yangjiang,Guangdong Chinese_tiger_beetles6-T tissue Yangjiang,Guangdong Chinese_tiger_beetles7-T tissue Yangjiang,Guangdong Chinese_tiger_beetles8-T tissue Yangjiang,Guangdong Chinese_tiger_beetles9-T tissue Yangjiang,Guangdong 4 Bombyx mori Silkworm1-T tissue Jinhua,Zhejiang Silkworm2-T tissue Jinhua,Zhejiang Silkworm3-T tissue Jinhua,Zhejiang Silkworm4-T tissue Jinhua,Zhejiang Silkworm5-T tissue Jinhua,Zhejiang Silkworm6-T tissue Jinhua,Zhejiang Silkworm7-T tissue Jinhua,Zhejiang Silkworm8-T tissue Jinhua,Zhejiang Silkworm9-T tissue Jinhua,Zhejiang Silkworm10-T tissue Jinhua,Zhejiang 5 Apis cerana Chinese_bee1-T tissue Quanzhou,Fujian Chinese_bee2-T -

A Biogeographic Synthesis of the Amphibians and Reptiles of Indochina
BAIN & HURLEY: AMPHIBIANS OF INDOCHINA & REPTILES & HURLEY: BAIN Scientific Publications of the American Museum of Natural History American Museum Novitates A BIOGEOGRAPHIC SYNTHESIS OF THE Bulletin of the American Museum of Natural History Anthropological Papers of the American Museum of Natural History AMPHIBIANS AND REPTILES OF INDOCHINA Publications Committee Robert S. Voss, Chair Board of Editors Jin Meng, Paleontology Lorenzo Prendini, Invertebrate Zoology RAOUL H. BAIN AND MARTHA M. HURLEY Robert S. Voss, Vertebrate Zoology Peter M. Whiteley, Anthropology Managing Editor Mary Knight Submission procedures can be found at http://research.amnh.org/scipubs All issues of Novitates and Bulletin are available on the web from http://digitallibrary.amnh.org/dspace Order printed copies from http://www.amnhshop.com or via standard mail from: American Museum of Natural History—Scientific Publications Central Park West at 79th Street New York, NY 10024 This paper meets the requirements of ANSI/NISO Z39.48-1992 (permanence of paper). AMNH 360 BULLETIN 2011 On the cover: Leptolalax sungi from Van Ban District, in northwestern Vietnam. Photo by Raoul H. Bain. BULLETIN OF THE AMERICAN MUSEUM OF NATURAL HISTORY A BIOGEOGRAPHIC SYNTHESIS OF THE AMPHIBIANS AND REPTILES OF INDOCHINA RAOUL H. BAIN Division of Vertebrate Zoology (Herpetology) and Center for Biodiversity and Conservation, American Museum of Natural History Life Sciences Section Canadian Museum of Nature, Ottawa, ON Canada MARTHA M. HURLEY Center for Biodiversity and Conservation, American Museum of Natural History Global Wildlife Conservation, Austin, TX BULLETIN OF THE AMERICAN MUSEUM OF NATURAL HISTORY Number 360, 138 pp., 9 figures, 13 tables Issued November 23, 2011 Copyright E American Museum of Natural History 2011 ISSN 0003-0090 CONTENTS Abstract......................................................... -

Interspecies Introgressive Hybridization in Spiny Frogs Quasipaa (Family Dicroglossidae) Revealed by Analyses on Multiple Mitochondrial and Nuclear Genes
Received: 12 June 2017 | Revised: 26 October 2017 | Accepted: 30 October 2017 DOI: 10.1002/ece3.3728 ORIGINAL RESEARCH Interspecies introgressive hybridization in spiny frogs Quasipaa (Family Dicroglossidae) revealed by analyses on multiple mitochondrial and nuclear genes Qi-Peng Zhang1,2 | Wen-Fang Hu1,2 | Ting-Ting Zhou1,2 | Shen-Shen Kong1,2 | Zhi-Fang Liu1,2 | Rong-Quan Zheng1,2,3 1Key Lab of Wildlife Biotechnology and Conservation and Utilization of Zhejiang Abstract Province, Jinhua, Zhejiang, China Introgression may lead to discordant patterns of variation among loci and traits. For 2 Institute of Ecology, Zhejiang Normal example, previous phylogeographic studies on the genus Quasipaa detected signs of University, Jinhua, Zhejiang, China genetic introgression from genetically and morphologically divergent Quasipaa shini or 3Xingzhi College of Zhejiang Normal University, Jinhua, Zhejiang, China Quasipaa spinosa. In this study, we used mitochondrial and nuclear DNA sequence data to verify the widespread introgressive hybridization in the closely related species Correspondence Rong-Quan Zheng, Key Lab of Wildlife of the genus Quasipaa, evaluate the level of genetic diversity, and reveal the formation Biotechnology and Conservation and mechanism of introgressive hybridization. In Longsheng, Guangxi Province, signs of Utilization of Zhejiang Province, Zhejiang Normal University, Jinhua, China. asymmetrical nuclear introgression were detected between Quasipaa boulengeri and Email: [email protected] Q. shini. Unidirectional mitochondrial introgression was revealed from Q. spinosa to Funding information Q. shini. By contrast, bidirectional mitochondrial gene introgression was detected be- National Natural Science Foundation of tween Q. spinosa and Q. shini in Lushan, Jiangxi Province. Our study also detected an- China, Grant/Award Number: 31472015 and 31172116; Key Program of Zhejiang cient hybridizations between a female Q. -

Species Groups Distributed Across Elevational Gradients Reveal Convergent and Continuous Genetic Adaptation to High Elevations
Species groups distributed across elevational gradients reveal convergent and continuous genetic adaptation to high elevations Yan-Bo Suna,1, Ting-Ting Fua,b,1, Jie-Qiong Jina, Robert W. Murphya,c, David M. Hillisd,2, Ya-Ping Zhanga,e,f,2, and Jing Chea,e,g,2 aState Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Sciences, 650223 Kunming, China; bKunming College of Life Science, University of Chinese Academy of Sciences, 650204 Kunming, China; cCentre for Biodiversity and Conservation Biology, Royal Ontario Museum, Toronto, ON M5S 2C6, Canada; dDepartment of Integrative Biology and Biodiversity Center, University of Texas at Austin, Austin, TX 78712; eCenter for Excellence in Animal Evolution and Genetics, Chinese Academy of Sciences, 650223 Kunming, China; fState Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Yunnan University, 650091 Kunming, China; and gSoutheast Asia Biodiversity Research Institute, Chinese Academy of Sciences, Yezin, 05282 Nay Pyi Taw, Myanmar Contributed by David M. Hillis, September 7, 2018 (sent for review August 7, 2018; reviewed by John H. Malone and Fuwen Wei) Although many cases of genetic adaptations to high elevations Most previous studies of the genetic processes of HEA have have been reported, the processes driving these modifications and compared species or populations from high elevations above the pace of their evolution remain unclear. Many high-elevation 3,500 m with those from low elevations to identify sequence adaptations (HEAs) are thought to have arisen in situ as popula- variation and/or expression shifts in the high-elevation group (8– tions rose with growing mountains. -

Climatic Niche Measures and Extinction Risks of Amphibians In
Australian Systematic Botany, 2017, 30, 414–421 ©CSIRO 2017 doi:10.1071/SB17001_AC Supplemental material Correlates of ecological-niche diversity and extinction risk of amphibians in China under climate change Youhua ChenA,C,D and Tania EscalanteB AChengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610000, P.R. China. BGrupo de Biogeografía de la Conservación, Departamento de Biología Evolutiva, Facultad de Ciencias, Universidad Nacional Autónoma de México, Circuito Exterior s/n, Ciudad Universitaria, Coyoacán, CP 04510, México. CDepartment of Renewable Resources, University of Alberta, Edmonton, AB, T6G 2H1, Canada. DCorresponding author. Email: [email protected] Page 1 of 12 Australian Systematic Botany ©CSIRO 2017 doi:10.1071/SB17001_AC Table S1. Correlations of different niche diversity metrics and the significance of the correlations Upper triangular matrix collects the correlation values, whereas the lower triangular matrix collects the P-values of the correlations. NC, niche connectivity; TNC, trend of NC; NB, niche breadth; TNB, trend of NB; NP, niche position; TNP, trend of NP; NA, niche amount; TNA, trend of NA; NCO, niche spatial correlation; TNCO, trend of NCO; NPA, niche packing; TNPA, trend of NPA; NFD, niche fractal dimension; TNFD, trend of NFD; NV, niche volume; and TNV, trend of NV NB NP NC NA NCO NPA NFD TNB TNP TNC TNA TNCO TNPA TNFD NB 1.000 0.515 0.263 0.088 0.335 –0.168 –0.071 –0.269 –0.056 –0.081 0.027 –0.086 0.095 –0.017 NP 0.000 1.000 0.003 –0.318 0.342 0.633 –0.423 –0.125 –0.134 –0.089 0.088 –0.151 -

Asia, Russia and Oceania
Issue number 104 (October 2012) ISSN: 1026-0269 eISSN: 1817-3934 Volume 20, number 5 www.amphibians.orgFrogLogConservation news for the herpetological community Regional Focus Asia, Russia and Oceania INSIDE News from the ASG Regional UpdatesAlytes, 2012, 29 (1¢4): 1-144. Recent Publications ISSN 0753-4973 General Announcements Contents Ariadne Angulo & Franco Andreone Bridging the gap between science and policy in amphibian conservation .............. 3-7 Simon N. Stuart And More... Responding to the amphibian crisis: too little, too late? ........................... 9-12 Action plans Claude Gascon, James P. Collins, Don R. Church, Robin D. Moore, Franco Andreone, NTERNATIONAL OURNAL OF ATRACHOLOGY Phil Bishop, Sathyabhama Das Biju, Federico Bolaños,Xie Feng, Li Pipeng, Li Zhang, I J B Haitao Shi, Stefan Lötters, Yolanda Matamoros, MadhavaRhacophorus Meegaskumbura, Sanjay Molur, rhodopus of Hainan Island, a beautiful treeforg from Indochina that Priya Nanjappa Mitchell, José Manuel Mora-Benavides, Jaime Garcia-Moreno, Herilala Randriamahazo, James T. Reardon, César Molina, Santiago Ron,JodiJ.L.Rowley, Débora Silvano, Paula H. Valdujo & Vanessa K. Verdade needs intact rainforest to survive. Photo credit: Bosco Chan@KCC. Scaling a global plan into regional strategies for amphibian conservation ............. 15-27 Vanessa K. Verdade, Paula H. Valdujo, Ana Carolina Carnaval, Luis Schiesari, Luís Felipe Toledo, Tami Mott, Gilda V. Andrade, Paula C. Eterovick, Marcelo Menin, Bruno V. S. Pimenta, Cristiano Nogueira, Cybele S. Lisboa, Cátia D. de Paula & Débora L. Silvano A leap further: the Brazilian Amphibian Conservation Action Plan .................. 28-43 Franco Andreone, Angus I. Carpenter, Jamieson Copsey, Angelica Crottini, Gerardo Garcia, Richard K. B. Jenkins, Jörn Köhler, Nirhy H. C. -
Complete Mitochondrial Genomes of Nanorana Taihangnica and N
Zhang et al. BMC Evolutionary Biology (2018) 18:26 https://doi.org/10.1186/s12862-018-1140-2 RESEARCH ARTICLE Open Access Complete mitochondrial genomes of Nanorana taihangnica and N. yunnanensis (Anura: Dicroglossidae) with novel gene arrangements and phylogenetic relationship of Dicroglossidae Jia-Yong Zhang1,2,3, Le-Ping Zhang2, Dan-Na Yu1,2* , Kenneth B. Storey3 and Rong-Quan Zheng4 Abstract Background: Complete mitochondrial (mt) genomes have been used extensively to test hypotheses about microevolution and to study population structure, phylogeography, and phylogenetic relationships of Anura at various taxonomic levels. Large-scale mt genomic reorganizations have been observed among many fork-tongued frogs (family Dicroglossidae). The relationships among Dicroglossidae and validation of the genus Feirana are still problematic. Hence, we sequenced the complete mt genomes of Nanorana taihangnica (=F. taihangnica)andN. yunnanensis as well as partial mt genomes of six Quasipaa species (dicroglossid taxa), two Odorrana and two Amolops species (Ranidae), and one Rhacophorus species (Rhacophoridae) in order to identify unknown mt gene rearrangements, to investigate the validity of the genus Feirana, and to test the phylogenetic relationship of Dicroglossidae. Results: In the mt genome of N. taihangnica two trnM genes, two trnP genes and two control regions were found. In addition, the trnA, trnN, trnC,andtrnQ genes were translocated from their typical positions. In the mt genome of N. yunnanensis, three control regions were found and eight genes (ND6, trnP, trnQ, trnA, trnN, trnC, trnY and trnS genes) in the L-stand were translocated from their typical position and grouped together. We also found intraspecific rearrangement of the mitochondrial genomes in N. -

Next-Generation Sequencing of Mixed Genomic DNA Allows Efficient Assembly of Rearranged Mitochondrial Genomes in Amolops Chunganensis and Quasipaa Boulengeri
Next-generation sequencing of mixed genomic DNA allows efficient assembly of rearranged mitochondrial genomes in Amolops chunganensis and Quasipaa boulengeri Siqi Yuan1,2, Yun Xia1, Yuchi Zheng1 and Xiaomao Zeng1 1 Department of Herpetology, Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu, Sichuan, China 2 University of Chinese Academy of Sciences, Beijing, China ABSTRACT Recent improvements in next-generation sequencing (NGS) technologies can facilitate the obtainment of mitochondrial genomes. However, it is not clear whether NGS could be effectively used to reconstruct the mitogenome with high gene rearrangement. These high rearrangements would cause amplification failure, and/or assembly and alignment errors. Here, we choose two frogs with rearranged gene order, Amolops chunganensis and Quasipaa boulengeri, to test whether gene rearrangements affect the mitogenome assembly and alignment by using NGS. The mitogenomes with gene rearrangements are sequenced through Illumina MiSeq genomic sequencing and assembled effectively by Trinity v2.1.0 and SOAPdenovo2. Gene order and contents in the mitogenome of A. chunganensis and Q. boulengeri are typical neobatrachian pattern except for rearrangements at the position of ``WANCY'' tRNA genes cluster. Further, the mitogenome of Q. boulengeri is characterized with a tandem duplication of trnM. Moreover, we utilize 13 protein-coding genes of A. chunganensis, Q. boulengeri and other neobatrachians to reconstruct the phylogenetic tree for evaluating mitochondrial sequence authenticity -

The Combined Effects of Temperature and Aromatase Inhibitor on Metamorphosis, Growth, Locomotion, and Sex Ratio of Tiger Frog (Hoplobatrachus Rugulosus) Tadpoles
The combined effects of temperature and aromatase inhibitor on metamorphosis, growth, locomotion, and sex ratio of tiger frog (Hoplobatrachus rugulosus) tadpoles Yun Tang1,2, Zhi-Qiang Chen1,3, You-Fu Lin1,4, Jing-Yi Chen1, Guo-Hua Ding1 and Xiang Ji2 1 Laboratory of Amphibian Diversity Investigation, College of Ecology, Lishui University, Lishui, Zhejiang, P.R. China 2 College of Life Sciences, Nanjing Normal University, Nanjing, Jiangsu, P.R. China 3 College of Animal Science and Technology, Zhejiang A & F University, Lin’an, Zhejiang, P.R. China 4 College of Forestry, Nanjing Forestry University, Nanjing, Jiangsu, P.R. China ABSTRACT Background: The tiger frog (Hoplobatrachus rugulosus) is widely raised by many farms in southern region of China as an economically edible frog. The growth, development, and sexual differentiation of amphibians are influenced by temperature and steroid hormone level. However, the problem of hormone residues is caused by the addition of exogenous hormones in frog breeding, it is worth considering whether non-sterol aromatase inhibitors can be used instead of hormones. Methods: In our study, H. rugulosus tadpoles were subjected to two water temperatures (29 C and 34 C) and three letrozole concentrations in the feed (0, 0.1 and 1 mg/g) to examine the effects of temperature, aromatase inhibitor and their interaction on metamorphosis, locomotion, and sex ratios. A G-test and contingency table were used to analyze the metamorphosis rate of tadpoles and the survival rate of froglets after feeding for 90 days. A G-test was also used to analyze sex ratios in different treatment groups. Submitted 10 July 2019 Results: Metamorphosis time and body size (snout–vent length, body mass and Accepted 2 March 2020 fi Published 20 March 2020 condition factor) were signi cantly different between the two temperature treatments. -

1 Appendix 6: Herpetofauna Sub-Group Report Status, Trends, and Recommendations on Hong Kong Herpetofauna A. Reptiles Overview
Appendix 6: Herpetofauna Sub-group Report Status, Trends, and Recommendations on Hong Kong Herpetofauna A. Reptiles Overview of status and trends Reptiles are a very diverse group with some 10,000 named species. In Hong Kong, five species of native freshwater turtles, five species of sea turtles, 21 species of lizards and over 50 species of snakes (including 6 sea snake species) have been recorded. The number of native snake species is not certain because two species, Dendrelaphis hollinrakei and Ahaetulla prasina medioxima reported by Lazell (2002) are based on old specimens without any collecting information and recent searches on the island where the specimens originated failed to find more record. Hence, their status in Hong Kong is not clear. Also one young snake found on Keung Shan, Lantau is so special that it cannot even be identified to the genus (Lazell, pers comm.). There are other species of uncertain status, either from old literature records or from specimens that might have been originated from trade and released locally. With over 80 native species, the reptile fauna in HK is exceptionally rich and includes about 25% of the species in China. Reptiles especially snakes are difficult to survey. Although reptiles have been studied for more than 60 years by naturalists and more recently by researchers and the Agriculture, Fisheries and Conservation Department (AFCD), our knowledge on many rare species is poor. There are still a number of little-known species with only a handful of local records. Species assessment of the local status could not be carried out due to time and manpower constraint and the AFCD extensive database was not available during the BSAP process.