Interspecific Hybridization of [I]Quasipaa[I] and Genetic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diet Composition and Overlap in a Montane Frog Community in Vietnam
Herpetological Conservation and Biology 13(1):205–215. Submitted: 5 November 2017; Accepted: 19 March 2018; Published 30 April 2018. DIET COMPOSITION AND OVERLAP IN A MONTANE FROG COMMUNITY IN VIETNAM DUONG THI THUY LE1,4, JODI J. L. ROWLEY2,3, DAO THI ANH TRAN1, THINH NGOC VO1, AND HUY DUC HOANG1 1Faculty of Biology and Biotechnology, University of Science, Vietnam National University-HCMC, 227 Nguyen Van Cu Street, District 5, Ho Chi Minh City, Vietnam 2Australian Museum Research Institute, Australian Museum,1 William Street, Sydney, New South Wales 2010, Australia 3Centre for Ecosystem Science, School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, New South Wales 2052, Australia 4Corresponding author, e-mail: [email protected] Abstract.—Southeast Asia is home to a highly diverse and endemic amphibian fauna under great threat. A significant obstacle to amphibian conservation prioritization in the region is a lack of basic biological information, including the diets of amphibians. We used stomach flushing to obtain data on diet composition, feeding strategies, dietary niche breadth, and overlap of nine species from a montane forest in Langbian Plateau, southern Vietnam: Feihyla palpebralis (Vietnamese Bubble-nest Frog), Hylarana montivaga (Langbian Plateau Frog), Indosylvirana milleti (Dalat Frog), Kurixalus baliogaster (Belly-spotted Frog), Leptobrachium pullum (Vietnam Spadefoot Toad), Limnonectes poilani (Poilane’s Frog), Megophrys major (Anderson’s Spadefoot Toad), Polypedates cf. leucomystax (Common Tree Frog), and Raorchestes gryllus (Langbian bubble-nest Frog). To assess food selectivity of these species, we sampled available prey in their environment. We classified prey items into 31 taxonomic groups. Blattodea was the dominant prey taxon for K. -

Is Dicroglossidae Anderson, 1871 (Amphibia, Anura) an Available Nomen?
Zootaxa 3838 (5): 590–594 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Correspondence ZOOTAXA Copyright © 2014 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3838.5.8 http://zoobank.org/urn:lsid:zoobank.org:pub:87DD8AF3-CB72-4EBD-9AA9-5B1E2439ABFE Is Dicroglossidae Anderson, 1871 (Amphibia, Anura) an available nomen? ANNEMARIE OHLER1 & ALAIN DUBOIS Muséum National d'Histoire Naturelle, Département Systématique et Evolution, UMR7205 ISYEB, CP 30, 25 rue Cuvier, 75005 Paris 1Corresponding autho. E-mail: [email protected] Abbreviations used: BMNH, Natural History Museum, London; SVL, snout–vent length; ZMB, Zoologisch Museum, Berlin. Anderson (1871a: 38) mentioned the family nomen DICROGLOSSIDAE, without any comment, in a list of specimens of the collections of the Indian Museum of Calcutta (now the Zoological Survey of India). He referred to this family a single species, Xenophrys monticola, a nomen given by Günther (1864) to a species of MEGOPHRYIDAE from Darjeeling and Khasi Hills (India) which has a complex nomenclatural history (Dubois 1989, 1992; Deuti et al. submitted). Dubois (1987: 57), considering that the nomen DICROGLOSSIDAE had been based on the generic nomen Dicroglossus Günther, 1860, applied it to a family group taxon, the tribe DICROGLOSSINI, for which he proposed a diagnosis. The genus Dicroglossus had been erected by Günther (1860), 11 years before Anderson’s (1871a) paper, for the unique species Dicroglossus adolfi. Boulenger (1882: 17) stated that this specific nomen was a subjective junior synonym of Rana cyanophlyctis Schneider, 1799, and therefore Dicroglossus a subjective junior synonym of Rana Linnaeus, 1758 (Boulenger, 1882: 7). -

Diet Composition, Body Condition and Sexual Size Dimorphism of the Common African
bioRxiv preprint doi: https://doi.org/10.1101/2021.01.25.428067; this version posted January 25, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Diet composition, body condition and sexual size dimorphism of the common African 2 toad (Amietophrynus regularis) in urban and agricultural landscape 3 Benjamin Yeboah Ofori*, John Bosu Mensah, Roger Sigismund Anderson and Daniel Korley 4 Attuquayefio 5 Department of Animal Biology and Conservation Science, University of Ghana, Legon 6 *Corresponding author; Email: [email protected] 7 8 9 Abstract 10 Land use and land cover change (LULCC) are major drivers of global biodiversity loss. The 11 conversion of natural habitats into human-modified landscapes poses novel and multifaceted 12 environmental stressors to organisms, influencing their ecology, physiology, life history and 13 fitness. Although the effects of LULCC have been studied extensively at the community level, 14 there is scant information about its effect on population and individual characteristics. We 15 assessed the diet composition, body condition, and sexual size dimorphism of the common 16 African toad (Amietophrynus regularis) in urban and agricultural landscape. Diet composition 17 was evaluated using gut content analysis, while body condition was measured using residual 18 mass index. Overall, 935 prey items comprising six classes, at least 18 orders and 31 families 19 were obtained from toads. This broad dietary niche suggested that Amietophrynus regularis is a 20 generalist predator. -

3Systematics and Diversity of Extant Amphibians
Systematics and Diversity of 3 Extant Amphibians he three extant lissamphibian lineages (hereafter amples of classic systematics papers. We present widely referred to by the more common term amphibians) used common names of groups in addition to scientifi c Tare descendants of a common ancestor that lived names, noting also that herpetologists colloquially refer during (or soon after) the Late Carboniferous. Since the to most clades by their scientifi c name (e.g., ranids, am- three lineages diverged, each has evolved unique fea- bystomatids, typhlonectids). tures that defi ne the group; however, salamanders, frogs, A total of 7,303 species of amphibians are recognized and caecelians also share many traits that are evidence and new species—primarily tropical frogs and salaman- of their common ancestry. Two of the most defi nitive of ders—continue to be described. Frogs are far more di- these traits are: verse than salamanders and caecelians combined; more than 6,400 (~88%) of extant amphibian species are frogs, 1. Nearly all amphibians have complex life histories. almost 25% of which have been described in the past Most species undergo metamorphosis from an 15 years. Salamanders comprise more than 660 species, aquatic larva to a terrestrial adult, and even spe- and there are 200 species of caecilians. Amphibian diver- cies that lay terrestrial eggs require moist nest sity is not evenly distributed within families. For example, sites to prevent desiccation. Thus, regardless of more than 65% of extant salamanders are in the family the habitat of the adult, all species of amphibians Plethodontidae, and more than 50% of all frogs are in just are fundamentally tied to water. -

Zoosystema 28(3)
Phylogenetic relationships and generic taxonomy of the tribe Paini (Amphibia, Anura, Ranidae, Dicroglossinae), with diagnoses of two new genera Annemarie OHLER Alain DUBOIS Muséum national d’Histoire naturelle, Département Systématique et Évolution, Reptiles et Amphibiens, USM 0602, case postale 30, 25 rue Cuvier, F-75231 Paris cedex 05 (France) [email protected] Ohler A. & Dubois A. 2006. — Phylogenetic relationships and generic taxonomy of the tribe Paini (Amphibia, Anura, Ranidae, Dicroglossinae), with diagnoses of two new genera. Zoosystema 28 (3) : 769-784. ABSTRACT KEY WORDS A preliminary cladistic analysis of the relationships between 26 frog species Amphibia, of the tribe Paini (Ranidae, Dicroglossinae) was carried out on the basis of 31 Anura, Ranidae, morphological characters, mainly from external morphology of adults. Combined Dicroglossinae, with the results of a molecular analysis published elsewhere, these data 1) confi rm Paini, that, after exclusion of the species Rana delacouri Angel, 1928, the Paini are a morphology, cladistic relationships, homophyletic group, and 2) allow to redefi ne the genera of this tribe, which new genera. are now six in number, including two new ones described herein. RÉSUMÉ Relations phylogénétiques et taxonomie générique de la tribu Paini (Amphibia, Anura, Ranidae, Dicroglossinae), avec diagnoses de deux nouveaux genres. Une analyse cladistique préliminaire des relations entre 26 espèces de grenouilles MOTS CLÉS de la tribu des Paini (Ranidae, Dicroglossinae) a été menée en utilisant 31 Amphibia, caractères morphologiques, principalement de la morphologie externe des Anura, Ranidae, adultes. Combinées avec les résultats d’une analyse moléculaire publiée ailleurs, Dicroglossinae, ces données 1) confi rment qu’après exclusion de l’espèce Rana delacouri Angel, Paini, 1928, les Paini constituent un groupe homophylétique, et 2) permettent de morphologie, analyse cladistique, redéfi nir les genres de cette tribu, qui sont maintenant au nombre de six, dont genres nouveaux. -

1704632114.Full.Pdf
Phylogenomics reveals rapid, simultaneous PNAS PLUS diversification of three major clades of Gondwanan frogs at the Cretaceous–Paleogene boundary Yan-Jie Fenga, David C. Blackburnb, Dan Lianga, David M. Hillisc, David B. Waked,1, David C. Cannatellac,1, and Peng Zhanga,1 aState Key Laboratory of Biocontrol, College of Ecology and Evolution, School of Life Sciences, Sun Yat-Sen University, Guangzhou 510006, China; bDepartment of Natural History, Florida Museum of Natural History, University of Florida, Gainesville, FL 32611; cDepartment of Integrative Biology and Biodiversity Collections, University of Texas, Austin, TX 78712; and dMuseum of Vertebrate Zoology and Department of Integrative Biology, University of California, Berkeley, CA 94720 Contributed by David B. Wake, June 2, 2017 (sent for review March 22, 2017; reviewed by S. Blair Hedges and Jonathan B. Losos) Frogs (Anura) are one of the most diverse groups of vertebrates The poor resolution for many nodes in anuran phylogeny is and comprise nearly 90% of living amphibian species. Their world- likely a result of the small number of molecular markers tra- wide distribution and diverse biology make them well-suited for ditionally used for these analyses. Previous large-scale studies assessing fundamental questions in evolution, ecology, and conser- used 6 genes (∼4,700 nt) (4), 5 genes (∼3,800 nt) (5), 12 genes vation. However, despite their scientific importance, the evolutionary (6) with ∼12,000 nt of GenBank data (but with ∼80% missing history and tempo of frog diversification remain poorly understood. data), and whole mitochondrial genomes (∼11,000 nt) (7). In By using a molecular dataset of unprecedented size, including 88-kb the larger datasets (e.g., ref. -

A De Novo Case of Floating Chromosomal Polymorphisms by Translocation in Quasipaa Boulengeri (Anura, Dicroglossidae)
A De Novo Case of Floating Chromosomal Polymorphisms by Translocation in Quasipaa boulengeri (Anura, Dicroglossidae) Liyan Qing1,2, Yun Xia1,2, Yuchi Zheng1, Xiaomao Zeng1* 1 Department of Herpetology, Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu, People’s Republic of China, 2 Graduate University of Chinese Academy of Sciences, Beijing, People’s Republic of China Abstract Very few natural polymorphisms involving interchromosomal reciprocal translocations are known in amphibians even in vertebrates. In this study, thirty three populations, including 471 individuals of the spiny frog Quasipaa boulengeri, were karyotypically examined using Giemsa stain or FISH. Five different karyomorphs were observed. The observed heteromorphism was autosomal but not sex-related, as the same heteromorphic chromosomes were found both in males and females. Our results indicated that the variant karyotypes resulted from a mutual interchange occurring between chromosomes 1 and 6. The occurrence of a nearly whole-arm translocation between chromosome no. 1 and no. 6 gave rise to a high frequency of alternate segregation and probably resulted in the maintenance of the translocation polymorphisms in a few populations. The translocation polymorphism is explained by different frequencies of segregation modes of the translocation heterozygote during meiosis. Theoretically, nine karyomorphs should be investigated, however, four expected karyotypes were not found. The absent karyomorphs may result from recessive lethal mutations, position effects, duplications and deficiencies. The phylogenetic inference proved that all populations of Q. boulengeri grouped into a monophyletic clade. The mutual translocation likely evolved just once in this species and the dispersal of the one karyomorph (type IV) can explain the chromosomal variations among populations. -

Progress and Prospects for Studies on Chinese Amphibians
Asian Herpetological Research 2010, 1(2): 64-85 DOI: 10.3724/SP.J.1245.2010.00064 Progress and Prospects for Studies on Chinese Amphibians FEI Liang, YE Changyuan and JIANG Jianping* Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, Sichuan, China Abstract This work summarizes the history and progress of the studies on Chinese amphibians since they first ap- peared in the Chinese literature. A wide range of research has been carried out, including the history of the definition of amphibians, faunal surveys, systematic research, ecological research, biochemical research (isozyme and other proteins or peptides, chromosomes, DNA), anatomical research, embryological research, phylogenetic and zoogeographical re- search, and many others such as ultrastructure of organs, crossbreeding test, regeneration of organs, abnormality survey, acoustics, fossils, sperm ultrastructure and parasites. In addition, the prospects for studies on Chinese amphibians in future are proposed in this paper. Keywords progress, prospect, faunal survey, systematics, amphibian, China 1. Introduction on amphibian research for at least 3000 years: toads (maybe Bufo gargarizans) were equated to ugliness and China is located in east Asia and covers a land area of wickedness in ‘The Book of Songs’ (-3000 years ago), approximately 9.6 million km2. Due to the vast territory but frog (鼃, 黽) had been inscribed on bones or tortoise occupied by this country, extremely different landforms, shell approximately 16th − 11th centuray B.C. (Guo et al., complex environments, and diverse climates and vegeta- 1999). “人鱼” (Mermen, now called the Chinese giant tion, China is very rich in amphibians, not only contai- salamander, Andrias davidianus), “活师” ( meaning tad- ning extremely numerous rare and endemic species, but poles), and “黾” (meaning frogs) were mentioned in ‘The also preserving a large number of relic species. -
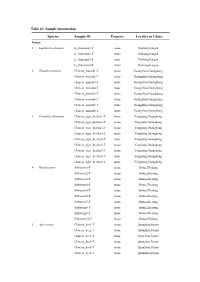
Table S1. Sample Information. Species Sample ID
Table S1. Sample information. Species Sample ID Property Locality in China Insects 1 Luehdorfia chinensis L_chinensis1-T tissue Nanking,Jiangsu L_chinensis2-T tissue Nanking,Jiangsu L_chinensis3-T tissue Nanking,Jiangsu L_chinensis4-T tissue Nanking,Jiangsu 2 Tenodera sinensis Chinese_mantid1-T tissue Guangzhou,Guangdong Chinese_mantid2-T tissue Guangzhou,Guangdong Chinese_mantid3-T tissue Guangzhou,Guangdong Chinese_mantid4-T tissue Guangzhou,Guangdong Chinese_mantid5-T tissue Guangzhou,Guangdong Chinese_mantid6-T tissue Guangzhou,Guangdong Chinese_mantid7-T tissue Guangzhou,Guangdong Chinese_mantid8-T tissue Guangzhou,Guangdong 3 Cicindela chinenesis Chinese_tiger_beetles1-T tissue Yangjiang,Guangdong Chinese_tiger_beetles2-T tissue Yangjiang,Guangdong Chinese_tiger_beetles3-T tissue Yangjiang,Guangdong Chinese_tiger_beetles4-T tissue Yangjiang,Guangdong Chinese_tiger_beetles5-T tissue Yangjiang,Guangdong Chinese_tiger_beetles6-T tissue Yangjiang,Guangdong Chinese_tiger_beetles7-T tissue Yangjiang,Guangdong Chinese_tiger_beetles8-T tissue Yangjiang,Guangdong Chinese_tiger_beetles9-T tissue Yangjiang,Guangdong 4 Bombyx mori Silkworm1-T tissue Jinhua,Zhejiang Silkworm2-T tissue Jinhua,Zhejiang Silkworm3-T tissue Jinhua,Zhejiang Silkworm4-T tissue Jinhua,Zhejiang Silkworm5-T tissue Jinhua,Zhejiang Silkworm6-T tissue Jinhua,Zhejiang Silkworm7-T tissue Jinhua,Zhejiang Silkworm8-T tissue Jinhua,Zhejiang Silkworm9-T tissue Jinhua,Zhejiang Silkworm10-T tissue Jinhua,Zhejiang 5 Apis cerana Chinese_bee1-T tissue Quanzhou,Fujian Chinese_bee2-T -

A Biogeographic Synthesis of the Amphibians and Reptiles of Indochina
BAIN & HURLEY: AMPHIBIANS OF INDOCHINA & REPTILES & HURLEY: BAIN Scientific Publications of the American Museum of Natural History American Museum Novitates A BIOGEOGRAPHIC SYNTHESIS OF THE Bulletin of the American Museum of Natural History Anthropological Papers of the American Museum of Natural History AMPHIBIANS AND REPTILES OF INDOCHINA Publications Committee Robert S. Voss, Chair Board of Editors Jin Meng, Paleontology Lorenzo Prendini, Invertebrate Zoology RAOUL H. BAIN AND MARTHA M. HURLEY Robert S. Voss, Vertebrate Zoology Peter M. Whiteley, Anthropology Managing Editor Mary Knight Submission procedures can be found at http://research.amnh.org/scipubs All issues of Novitates and Bulletin are available on the web from http://digitallibrary.amnh.org/dspace Order printed copies from http://www.amnhshop.com or via standard mail from: American Museum of Natural History—Scientific Publications Central Park West at 79th Street New York, NY 10024 This paper meets the requirements of ANSI/NISO Z39.48-1992 (permanence of paper). AMNH 360 BULLETIN 2011 On the cover: Leptolalax sungi from Van Ban District, in northwestern Vietnam. Photo by Raoul H. Bain. BULLETIN OF THE AMERICAN MUSEUM OF NATURAL HISTORY A BIOGEOGRAPHIC SYNTHESIS OF THE AMPHIBIANS AND REPTILES OF INDOCHINA RAOUL H. BAIN Division of Vertebrate Zoology (Herpetology) and Center for Biodiversity and Conservation, American Museum of Natural History Life Sciences Section Canadian Museum of Nature, Ottawa, ON Canada MARTHA M. HURLEY Center for Biodiversity and Conservation, American Museum of Natural History Global Wildlife Conservation, Austin, TX BULLETIN OF THE AMERICAN MUSEUM OF NATURAL HISTORY Number 360, 138 pp., 9 figures, 13 tables Issued November 23, 2011 Copyright E American Museum of Natural History 2011 ISSN 0003-0090 CONTENTS Abstract......................................................... -

Interspecies Introgressive Hybridization in Spiny Frogs Quasipaa (Family Dicroglossidae) Revealed by Analyses on Multiple Mitochondrial and Nuclear Genes
Received: 12 June 2017 | Revised: 26 October 2017 | Accepted: 30 October 2017 DOI: 10.1002/ece3.3728 ORIGINAL RESEARCH Interspecies introgressive hybridization in spiny frogs Quasipaa (Family Dicroglossidae) revealed by analyses on multiple mitochondrial and nuclear genes Qi-Peng Zhang1,2 | Wen-Fang Hu1,2 | Ting-Ting Zhou1,2 | Shen-Shen Kong1,2 | Zhi-Fang Liu1,2 | Rong-Quan Zheng1,2,3 1Key Lab of Wildlife Biotechnology and Conservation and Utilization of Zhejiang Abstract Province, Jinhua, Zhejiang, China Introgression may lead to discordant patterns of variation among loci and traits. For 2 Institute of Ecology, Zhejiang Normal example, previous phylogeographic studies on the genus Quasipaa detected signs of University, Jinhua, Zhejiang, China genetic introgression from genetically and morphologically divergent Quasipaa shini or 3Xingzhi College of Zhejiang Normal University, Jinhua, Zhejiang, China Quasipaa spinosa. In this study, we used mitochondrial and nuclear DNA sequence data to verify the widespread introgressive hybridization in the closely related species Correspondence Rong-Quan Zheng, Key Lab of Wildlife of the genus Quasipaa, evaluate the level of genetic diversity, and reveal the formation Biotechnology and Conservation and mechanism of introgressive hybridization. In Longsheng, Guangxi Province, signs of Utilization of Zhejiang Province, Zhejiang Normal University, Jinhua, China. asymmetrical nuclear introgression were detected between Quasipaa boulengeri and Email: [email protected] Q. shini. Unidirectional mitochondrial introgression was revealed from Q. spinosa to Funding information Q. shini. By contrast, bidirectional mitochondrial gene introgression was detected be- National Natural Science Foundation of tween Q. spinosa and Q. shini in Lushan, Jiangxi Province. Our study also detected an- China, Grant/Award Number: 31472015 and 31172116; Key Program of Zhejiang cient hybridizations between a female Q. -

Species Groups Distributed Across Elevational Gradients Reveal Convergent and Continuous Genetic Adaptation to High Elevations
Species groups distributed across elevational gradients reveal convergent and continuous genetic adaptation to high elevations Yan-Bo Suna,1, Ting-Ting Fua,b,1, Jie-Qiong Jina, Robert W. Murphya,c, David M. Hillisd,2, Ya-Ping Zhanga,e,f,2, and Jing Chea,e,g,2 aState Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Sciences, 650223 Kunming, China; bKunming College of Life Science, University of Chinese Academy of Sciences, 650204 Kunming, China; cCentre for Biodiversity and Conservation Biology, Royal Ontario Museum, Toronto, ON M5S 2C6, Canada; dDepartment of Integrative Biology and Biodiversity Center, University of Texas at Austin, Austin, TX 78712; eCenter for Excellence in Animal Evolution and Genetics, Chinese Academy of Sciences, 650223 Kunming, China; fState Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Yunnan University, 650091 Kunming, China; and gSoutheast Asia Biodiversity Research Institute, Chinese Academy of Sciences, Yezin, 05282 Nay Pyi Taw, Myanmar Contributed by David M. Hillis, September 7, 2018 (sent for review August 7, 2018; reviewed by John H. Malone and Fuwen Wei) Although many cases of genetic adaptations to high elevations Most previous studies of the genetic processes of HEA have have been reported, the processes driving these modifications and compared species or populations from high elevations above the pace of their evolution remain unclear. Many high-elevation 3,500 m with those from low elevations to identify sequence adaptations (HEAs) are thought to have arisen in situ as popula- variation and/or expression shifts in the high-elevation group (8– tions rose with growing mountains.