H2 Reduction Zn (S) + 2 H+ → Zn2+ + H2 Redox R
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Elements of Electrochemistry
Page 1 of 8 Chem 201 Winter 2006 ELEM ENTS OF ELEC TROCHEMIS TRY I. Introduction A. A number of analytical techniques are based upon oxidation-reduction reactions. B. Examples of these techniques would include: 1. Determinations of Keq and oxidation-reduction midpoint potentials. 2. Determination of analytes by oxidation-reductions titrations. 3. Ion-specific electrodes (e.g., pH electrodes, etc.) 4. Gas-sensing probes. 5. Electrogravimetric analysis: oxidizing or reducing analytes to a known product and weighing the amount produced 6. Coulometric analysis: measuring the quantity of electrons required to reduce/oxidize an analyte II. Terminology A. Reduction: the gaining of electrons B. Oxidation: the loss of electrons C. Reducing agent (reductant): species that donates electrons to reduce another reagent. (The reducing agent get oxidized.) D. Oxidizing agent (oxidant): species that accepts electrons to oxidize another species. (The oxidizing agent gets reduced.) E. Oxidation-reduction reaction (redox reaction): a reaction in which electrons are transferred from one reactant to another. 1. For example, the reduction of cerium(IV) by iron(II): Ce4+ + Fe2+ ! Ce3+ + Fe3+ a. The reduction half-reaction is given by: Ce4+ + e- ! Ce3+ b. The oxidation half-reaction is given by: Fe2+ ! e- + Fe3+ 2. The half-reactions are the overall reaction broken down into oxidation and reduction steps. 3. Half-reactions cannot occur independently, but are used conceptually to simplify understanding and balancing the equations. III. Rules for Balancing Oxidation-Reduction Reactions A. Write out half-reaction "skeletons." Page 2 of 8 Chem 201 Winter 2006 + - B. Balance the half-reactions by adding H , OH or H2O as needed, maintaining electrical neutrality. -

All-Carbon Electrodes for Flexible Solar Cells
applied sciences Article All-Carbon Electrodes for Flexible Solar Cells Zexia Zhang 1,2,3 ID , Ruitao Lv 1,2,*, Yi Jia 4, Xin Gan 1,5 ID , Hongwei Zhu 1,2 and Feiyu Kang 1,5,* 1 State Key Laboratory of New Ceramics and Fine Processing, School of Materials Science and Engineering, Tsinghua University, Beijing 100084, China; [email protected] (Z.Z.); [email protected] (X.G.); [email protected] (H.Z.) 2 Key Laboratory of Advanced Materials (MOE), School of Materials Science and Engineering, Tsinghua University, Beijing 100084, China 3 School of Physics and Electronic Engineering, Xinjiang Normal University, Urumqi 830046, Xinjiang Province, China 4 Qian Xuesen Laboratory of Space Technology, China Academy of Space Technology, Beijing 100094, China; [email protected] 5 Graduate School at Shenzhen, Tsinghua University, Shenzhen 518055, Guangdong Province, China * Correspondences: [email protected] (R.L.); [email protected] (F.K.) Received: 16 December 2017; Accepted: 20 January 2018; Published: 23 January 2018 Abstract: Transparent electrodes based on carbon nanomaterials have recently emerged as new alternatives to indium tin oxide (ITO) or noble metal in organic photovoltaics (OPVs) due to their attractive advantages, such as long-term stability, environmental friendliness, high conductivity, and low cost. However, it is still a challenge to apply all-carbon electrodes in OPVs. Here, we report our efforts to develop all-carbon electrodes in organic solar cells fabricated with different carbon-based materials, including carbon nanotubes (CNTs) and graphene films synthesized by chemical vapor deposition (CVD). Flexible and semitransparent solar cells with all-carbon electrodes are successfully fabricated. -

A Review of Cathode and Anode Materials for Lithium-Ion Batteries
A Review of Cathode and Anode Materials for Lithium-Ion Batteries Yemeserach Mekonnen Aditya Sundararajan Arif I. Sarwat IEEE Student Member IEEE Student Member IEEE Member Department of Electrical & Department of Electrical & Department of Electrical & Computer Engineering Computer Engineering Computer Engineering Florida International University Florida International University Florida International University Email: [email protected] Email: [email protected] Email: [email protected] Abstract—Lithium ion batteries are one of the most technologies such as plug-in HEVs. For greater application use, commercially sought after energy storages today. Their batteries are usually expensive and heavy. Li-ion and Li- based application widely spans from Electric Vehicle (EV) to portable batteries show promising advantages in creating smaller, devices. Their lightness and high energy density makes them lighter and cheaper battery storage for such high-end commercially viable. More research is being conducted to better applications [18]. As a result, these batteries are widely used in select the materials for the anode and cathode parts of Lithium (Li) ion cell. This paper presents a comprehensive review of the common consumer electronics and account for higher sale existing and potential developments in the materials used for the worldwide [2]. Lithium, as the most electropositive element making of the best cathodes, anodes and electrolytes for the Li- and the lightest metal, is a unique element for the design of ion batteries such that maximum efficiency can be tapped. higher density energy storage systems. The discovery of Observed challenges in selecting the right set of materials is also different inorganic compounds that react with alkali metals in a described in detail. -

Galvanic Cell Notation • Half-Cell Notation • Types of Electrodes • Cell
Galvanic Cell Notation ¾Inactive (inert) electrodes – not involved in the electrode half-reaction (inert solid conductors; • Half-cell notation serve as a contact between the – Different phases are separated by vertical lines solution and the external el. circuit) 3+ 2+ – Species in the same phase are separated by Example: Pt electrode in Fe /Fe soln. commas Fe3+ + e- → Fe2+ (as reduction) • Types of electrodes Notation: Fe3+, Fe2+Pt(s) ¾Active electrodes – involved in the electrode ¾Electrodes involving metals and their half-reaction (most metal electrodes) slightly soluble salts Example: Zn2+/Zn metal electrode Example: Ag/AgCl electrode Zn(s) → Zn2+ + 2e- (as oxidation) AgCl(s) + e- → Ag(s) + Cl- (as reduction) Notation: Zn(s)Zn2+ Notation: Cl-AgCl(s)Ag(s) ¾Electrodes involving gases – a gas is bubbled Example: A combination of the Zn(s)Zn2+ and over an inert electrode Fe3+, Fe2+Pt(s) half-cells leads to: Example: H2 gas over Pt electrode + - H2(g) → 2H + 2e (as oxidation) + Notation: Pt(s)H2(g)H • Cell notation – The anode half-cell is written on the left of the cathode half-cell Zn(s) → Zn2+ + 2e- (anode, oxidation) + – The electrodes appear on the far left (anode) and Fe3+ + e- → Fe2+ (×2) (cathode, reduction) far right (cathode) of the notation Zn(s) + 2Fe3+ → Zn2+ + 2Fe2+ – Salt bridges are represented by double vertical lines ⇒ Zn(s)Zn2+ || Fe3+, Fe2+Pt(s) 1 + Example: A combination of the Pt(s)H2(g)H Example: Write the cell reaction and the cell and Cl-AgCl(s)Ag(s) half-cells leads to: notation for a cell consisting of a graphite cathode - 2+ Note: The immersed in an acidic solution of MnO4 and Mn 4+ reactants in the and a graphite anode immersed in a solution of Sn 2+ overall reaction are and Sn . -

Demonstrating Solar Conversion Using Natural Dye Sensitizers
Demonstrating Solar Conversion Using Natural Dye Sensitizers Subject Area(s) Science & Technology, Physical Science, Environmental Science, Physics, Biology, and Chemistry Associated Unit Renewable Energy Lesson Title Dye Sensitized Solar Cell (DSSC) Grade Level (11th-12th) Time Required 3 hours / 3 day lab Summary Students will analyze the use of solar energy, explore future trends in solar, and demonstrate electron transfer by constructing a dye-sensitized solar cell using vegetable and fruit products. Students will analyze how energy is measured and test power output from their solar cells. Engineering Connection and Tennessee Careers An important aspect of building solar technology is the study of the type of materials that conduct electricity and understanding the reason why they conduct electricity. Within the TN-SCORE program Chemical Engineers, Biologist, Physicist, and Chemists are working together to provide innovative ways for sustainable improvements in solar energy technologies. The lab for this lesson is designed so that students apply their scientific discoveries in solar design. Students will explore how designing efficient and cost effective solar panels and fuel cells will respond to the social, political, and economic needs of society today. Teachers can use the Metropolitan Policy Program Guide “Sizing The Clean Economy: State of Tennessee” for information on Clean Economy Job Growth, TN Clean Economy Profile, and Clean Economy Employers. www.brookings.edu/metro/clean_economy.aspx Keywords Photosynthesis, power, electricity, renewable energy, solar cells, photovoltaic (PV), chlorophyll, dye sensitized solar cells (DSSC) Page 1 of 10 Next Generation Science Standards HS.ESS-Climate Change and Human Sustainability HS.PS-Chemical Reactions, Energy, Forces and Energy, and Nuclear Processes HS.ETS-Engineering Design HS.ETS-ETSS- Links Among Engineering, Technology, Science, and Society Pre-Requisite Knowledge Vocabulary: Catalyst- A substance that increases the rate of reaction without being consumed in the reaction. -

Chapter 13: Electrochemical Cells
March 19, 2015 Chapter 13: Electrochemical Cells electrochemical cell: any device that converts chemical energy into electrical energy, or vice versa March 19, 2015 March 19, 2015 Voltaic Cell -any device that uses a redox reaction to transform chemical potential energy into electrical energy (moving electrons) -the oxidizing agent and reducing agent are separated -each is contained in a half cell There are two half cells in a voltaic cell Cathode Anode -contains the SOA -contains the SRA -reduction reaction -oxidation takes place takes place - (-) electrode -+ electrode -anions migrate -cations migrate towards the anode towards cathode March 19, 2015 Electrons move through an external circuit from the anode to cathode Electricity is produced by the cell until one of the reactants is used up Example: A simple voltaic cell March 19, 2015 When designing half cells it is important to note the following: -each half cell needs an electrolyte and a solid conductor -the electrode and electrolyte cannot react spontaneously with each other (sometimes carbon and platinum are used as inert electrodes) March 19, 2015 There are two kinds of porous boundaries 1. Salt Bridge 2. Porous Cup · an unglazed ceramic cup · tube filled with an inert · separates solutions but electrolyte such as NaNO allows ions to pass 3 through or Na2SO4 · the ends are plugged so the solutions are separated, but ions can pass through Porous boundaries allow for ions to move between two half cells so that charge can be equalized between two half cells 2+ 2– electrolyte: Cu (aq), SO4 (aq) 2+ 2– electrolyte: Zn (aq), SO4 (aq) electrode: zinc electrode: copper March 19, 2015 Example: Metal/Ion Voltaic Cell V Co(s) Zn(s) Co2+ SO 2- 4 2+ SO 2- Zn 4 Example: A voltaic cell with an inert electrode March 19, 2015 Example Label the cathode, anode, electron movement, ion movement, and write the half reactions taking place at each half cell. -

Operation, Manufacturing, and Supply Chain of Lithium-Ion Batteries for Electric Vehicles
ORNL/TM-2020/1729 Operation, Manufacturing, and Supply Chain of Lithium-ion Batteries for Electric Vehicles Ilias Belharouak Jagjit Nanda Ethan Self W. Blake Hawley David Wood Zhijia Du Jianlin Li Approved for public release. Ronald L. Graves Distribution is unlimited. August 2020 DOCUMENT AVAILABILITY Reports produced after January 1, 1996, are generally available free via US Department of Energy (DOE) SciTech Connect. Website www.osti.gov Reports produced before January 1, 1996, may be purchased by members of the public from the following source: National Technical Information Service 5285 Port Royal Road Springfield, VA 22161 Telephone 703-605-6000 (1-800-553-6847) TDD 703-487-4639 Fax 703-605-6900 E-mail [email protected] Website http://classic.ntis.gov/ Reports are available to DOE employees, DOE contractors, Energy Technology Data Exchange representatives, and International Nuclear Information System representatives from the following source: Office of Scientific and Technical Information PO Box 62 Oak Ridge, TN 37831 Telephone 865-576-8401 Fax 865-576-5728 E-mail [email protected] Website http://www.osti.gov/contact.html This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. -

Electrochemistry –An Oxidizing Agent Is a Species That Oxidizes Another Species; It Is Itself Reduced
Oxidation-Reduction Reactions Chapter 17 • Describing Oxidation-Reduction Reactions Electrochemistry –An oxidizing agent is a species that oxidizes another species; it is itself reduced. –A reducing agent is a species that reduces another species; it is itself oxidized. Loss of 2 e-1 oxidation reducing agent +2 +2 Fe( s) + Cu (aq) → Fe (aq) + Cu( s) oxidizing agent Gain of 2 e-1 reduction Skeleton Oxidation-Reduction Equations Electrochemistry ! Identify what species is being oxidized (this will be the “reducing agent”) ! Identify what species is being •The study of the interchange of reduced (this will be the “oxidizing agent”) chemical and electrical energy. ! What species result from the oxidation and reduction? ! Does the reaction occur in acidic or basic solution? 2+ - 3+ 2+ Fe (aq) + MnO4 (aq) 6 Fe (aq) + Mn (aq) Steps in Balancing Oxidation-Reduction Review of Terms Equations in Acidic solutions 1. Assign oxidation numbers to • oxidation-reduction (redox) each atom so that you know reaction: involves a transfer of what is oxidized and what is electrons from the reducing agent to reduced 2. Split the skeleton equation into the oxidizing agent. two half-reactions-one for the oxidation reaction (element • oxidation: loss of electrons increases in oxidation number) and one for the reduction (element decreases in oxidation • reduction: gain of electrons number) 2+ 3+ - 2+ Fe (aq) º Fe (aq) MnO4 (aq) º Mn (aq) 1 3. Complete and balance each half reaction Galvanic Cell a. Balance all atoms except O and H 2+ 3+ - 2+ (Voltaic Cell) Fe (aq) º Fe (aq) MnO4 (aq) º Mn (aq) b. -

Battery Technologies for Small Scale Embeded Generation
Battery Technologies for Small Scale Embedded Generation. by Norman Jackson, South African Energy Storage Association (SAESA) Content Provider – Wikipedia et al Small Scale Embedded Generation - SSEG • SSEG is very much a local South African term for Distributed Generation under 10 Mega Watt. Internationally they refer to: Distributed generation, also distributed energy, on-site generation (OSG) or district/decentralized energy It is electrical generation and storage performed by a variety of small, grid- connected devices referred to as distributed energy resources (DER) Types of Energy storage: • Fossil fuel storage • Thermal • Electrochemical • Mechanical • Brick storage heater • Compressed air energy storage • Cryogenic energy storage (Battery Energy • Fireless locomotive • Liquid nitrogen engine Storage System, • Flywheel energy storage • Eutectic system BESS) • Gravitational potential energy • Ice storage air conditioning • Hydraulic accumulator • Molten salt storage • Flow battery • Pumped-storage • Phase-change material • Rechargeable hydroelectricity • Seasonal thermal energy battery • Electrical, electromagnetic storage • Capacitor • Solar pond • UltraBattery • Supercapacitor • Steam accumulator • Superconducting magnetic • Thermal energy energy storage (SMES, also storage (general) superconducting storage coil) • Chemical • Biological • Biofuels • Glycogen • Hydrated salts • Starch • Hydrogen storage • Hydrogen peroxide • Power to gas • Vanadium pentoxide History of the battery This was a stack of copper and zinc Italian plates, -

Effect of Stratification of Cathode Catalyst Layers on Durability of Proton Exchange Membrane Fuel Cells
energies Article Effect of Stratification of Cathode Catalyst Layers on Durability of Proton Exchange Membrane Fuel Cells Zikhona Nondudule 1, Jessica Chamier 2,* and Mahabubur Chowdhury 1 1 Department of Chemical Engineering, Faculty of Engineering, Cape Peninsula University of Technology, Cape Town 7530, South Africa; [email protected] (Z.N.); [email protected] (M.C.) 2 Department of Chemical Engineering, Centre for Catalysis Research, HySA Catalysis, University of Cape Town, Cape Town 7700, South Africa * Correspondence: [email protected]; Tel.: +27-(0)-21-650-2515 Abstract: To decrease the cost of fuel cell manufacturing, the amount of platinum (Pt) in the catalyst layer needs to be reduced. In this study, ionomer gradient membrane electrode assemblies (MEAs) were designed to reduce Pt loading without sacrificing performance and lifetime. A two-layer stratification of the cathode was achieved with varying ratios of 28 wt. % ionomer in the inner layer, on the membrane, and 24 wt. % on the outer layer, coated onto the inner layer. To study the MEA performance, the electrochemical surface area (ECSA), polarization curves, and electrochemical impedance spectroscopy (EIS) responses were evaluated under 20, 60, and 100% relative humidity (RH). The stratified MEA Pt loading was reduced by 12% while maintaining commercial equivalent performance. The optimal two-layer design was achieved when the Pt loading ratio between the layers was 1:6 (inner:outer layer). This MEA showed the highest ECSA and performance at 0.65 V with reduced mass transport losses. The integrity of stratified MEAs with lower Pt loading was evaluated with potential cycling and proved more durable than the monolayer MEA equivalent. -

2020 Grid Energy Storage Technology Cost and Performance Assessment
Energy Storage Grand Challenge Cost and Performance Assessment 2020 December 2020 2020 Grid Energy Storage Technology Cost and Performance Assessment Kendall Mongird, Vilayanur Viswanathan, Jan Alam, Charlie Vartanian, Vincent Sprenkle*, Pacific Northwest National LaBoratory. Richard Baxter, Mustang Prairie Energy * [email protected] Technical Report Publication No. DOE/PA-0204 December 2020 Energy Storage Grand Challenge Cost and Performance Assessment 2020 December 2020 Disclaimer This report was prepared as an account of work sponsored by an agency of the United States government. Neither the United States government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States government. ii Energy Storage Grand Challenge Cost and Performance Assessment 2020 December 2020 Acronyms AC alternating current Ah ampere-hour BESS battery energy storage system BLS U.S. Bureau of Labor Statistics BMS battery management system BOP balance of plant BOS balance of system C&C controls & communication C&I civil and infrastructure CAES compressed-air energy -
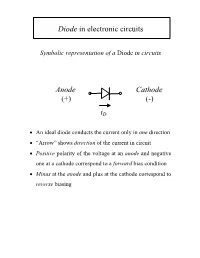
Diode in Electronic Circuits Anode Cathode (+) (-) Id
Diode in electronic circuits Symbolic representation of a Diode in circuits Anode Cathode (+) (-) iD • An ideal diode conducts the current only in one direction • “Arrow” shows direction of the current in circuit • Positive polarity of the voltage at an anode and negative one at a cathode correspond to a forward bias condition • Minus at the anode and plus at the cathode correspond to reverse biasing Diode as a non-linear resistor Current-Voltage (I-V) Characteristics I-V of a LINEAR RESISTOR i(A) R=v/i v(V) I-V of an IDEALIZED DIODE i(A) R → 0 v(V) R →∞ I-V Characteristic of an Ideal Diode qV /kT JJ=−S ()e 1 i(A) ~ exp(qV/kT) v(V) • At room temperature T=300K the thermal voltage kT/q = 26 meV • For a Si diode the typical value of the saturation current -10 2 JS ~ 10 A/cm JJ≈ eqV /kT For a high forward bias V >> 26 mV: S • The forward current shows an exponential dependence For a high reverse bias V <0, V< < -26 mV: J ≈ −J S • Reverse current of diodes is quite small I-V Characteristic of a Diode in semilogarithmic scale J(A/cm2) non-exp 102 increase 10-2 saturation slope -1 region 10-6 q/nkT~ 20-40 V |J | S 10-10 -10 10 20 30 qV/kT • The slop in logarithmic scale can be used to define the non-ideality factor n • The intersection of lgI-V characteristic with vertical axis gives the value of saturation current JS • The current increase differs from exponential at high forward bias voltage I-V Characteristic of a Real Diode A Silicon (Si) Diode i(A) v(V) 0.7 V • The typical voltage drop across a Si diode at forward bias is 0.7V A Germanium