Glenn Seaborg Never Forgot His Roots in Ishpeming and Was Always Very Proud of His Swedish Ancestry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ira Sprague Bowen Papers, 1940-1973
http://oac.cdlib.org/findaid/ark:/13030/tf2p300278 No online items Inventory of the Ira Sprague Bowen Papers, 1940-1973 Processed by Ronald S. Brashear; machine-readable finding aid created by Gabriela A. Montoya Manuscripts Department The Huntington Library 1151 Oxford Road San Marino, California 91108 Phone: (626) 405-2203 Fax: (626) 449-5720 Email: [email protected] URL: http://www.huntington.org/huntingtonlibrary.aspx?id=554 © 1998 The Huntington Library. All rights reserved. Observatories of the Carnegie Institution of Washington Collection Inventory of the Ira Sprague 1 Bowen Papers, 1940-1973 Observatories of the Carnegie Institution of Washington Collection Inventory of the Ira Sprague Bowen Paper, 1940-1973 The Huntington Library San Marino, California Contact Information Manuscripts Department The Huntington Library 1151 Oxford Road San Marino, California 91108 Phone: (626) 405-2203 Fax: (626) 449-5720 Email: [email protected] URL: http://www.huntington.org/huntingtonlibrary.aspx?id=554 Processed by: Ronald S. Brashear Encoded by: Gabriela A. Montoya © 1998 The Huntington Library. All rights reserved. Descriptive Summary Title: Ira Sprague Bowen Papers, Date (inclusive): 1940-1973 Creator: Bowen, Ira Sprague Extent: Approximately 29,000 pieces in 88 boxes Repository: The Huntington Library San Marino, California 91108 Language: English. Provenance Placed on permanent deposit in the Huntington Library by the Observatories of the Carnegie Institution of Washington Collection. This was done in 1989 as part of a letter of agreement (dated November 5, 1987) between the Huntington and the Carnegie Observatories. The papers have yet to be officially accessioned. Cataloging of the papers was completed in 1989 prior to their transfer to the Huntington. -

Copyright by Paul Harold Rubinson 2008
Copyright by Paul Harold Rubinson 2008 The Dissertation Committee for Paul Harold Rubinson certifies that this is the approved version of the following dissertation: Containing Science: The U.S. National Security State and Scientists’ Challenge to Nuclear Weapons during the Cold War Committee: —————————————————— Mark A. Lawrence, Supervisor —————————————————— Francis J. Gavin —————————————————— Bruce J. Hunt —————————————————— David M. Oshinsky —————————————————— Michael B. Stoff Containing Science: The U.S. National Security State and Scientists’ Challenge to Nuclear Weapons during the Cold War by Paul Harold Rubinson, B.A.; M.A. Dissertation Presented to the Faculty of the Graduate School of The University of Texas at Austin in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy The University of Texas at Austin August 2008 Acknowledgements Thanks first and foremost to Mark Lawrence for his guidance, support, and enthusiasm throughout this project. It would be impossible to overstate how essential his insight and mentoring have been to this dissertation and my career in general. Just as important has been his camaraderie, which made the researching and writing of this dissertation infinitely more rewarding. Thanks as well to Bruce Hunt for his support. Especially helpful was his incisive feedback, which both encouraged me to think through my ideas more thoroughly, and reined me in when my writing overshot my argument. I offer my sincerest gratitude to the Smith Richardson Foundation and Yale University International Security Studies for the Predoctoral Fellowship that allowed me to do the bulk of the writing of this dissertation. Thanks also to the Brady-Johnson Program in Grand Strategy at Yale University, and John Gaddis and the incomparable Ann Carter-Drier at ISS. -

Otto Hahn Otto Hahn
R.N. 70269/98 Postal Registration No.: DL-SW-1/4082/15-17 ISSN : 0972-169X Date of posting: 26-27 of advance month Date of publication: 24 of advance month May 2017 Vol. 19 No. 8 Rs. 5.00 Otto Hahn Discoverer of Nuclear Fission Editorial: Consolidating 35 science communication activities in our country Otto Hahn: Discoverer of 34 Nuclear Fission Keep Your Eyes Healthy 31 Phenol: A Serious 30 Environmental Threat Accidental Discoveries in 28 Medical Science Cures for haemorrhoids— 24 Simple treatments and Surgeries Recent developments 21 in science and technology 36 Editorial Consolidating science communication activities in our country Dr. R. Gopichandran It is well known that the National Council of Science Museums of India’s leadership in science technology and innovation (STI) across the Ministry of Culture, Government of India, the National Institute the bilateral and multilateral framework also. The news feature service of Science Communication and Information Resources (NISCAIR) and the portal activity have well defined action plans to reach out to of CSIR, the National Council for Science and Technology fellow institutions and citizens with suitably embellished platform Communication (NCSTC) of the Department of Science and and opportunities for all to deliver together. Technology (DST), Government of India and Vigyan Prasar, also While these are interesting and extremely important, especially of DST, have been carrying out excellent science communication because they respond to the call to upscale and value add science activities over the years. It cannot be denied that the reach has been and technology communication, it is equally important to document quite significant collectively. -

Appendix E Nobel Prizes in Nuclear Science
Nuclear Science—A Guide to the Nuclear Science Wall Chart ©2018 Contemporary Physics Education Project (CPEP) Appendix E Nobel Prizes in Nuclear Science Many Nobel Prizes have been awarded for nuclear research and instrumentation. The field has spun off: particle physics, nuclear astrophysics, nuclear power reactors, nuclear medicine, and nuclear weapons. Understanding how the nucleus works and applying that knowledge to technology has been one of the most significant accomplishments of twentieth century scientific research. Each prize was awarded for physics unless otherwise noted. Name(s) Discovery Year Henri Becquerel, Pierre Discovered spontaneous radioactivity 1903 Curie, and Marie Curie Ernest Rutherford Work on the disintegration of the elements and 1908 chemistry of radioactive elements (chem) Marie Curie Discovery of radium and polonium 1911 (chem) Frederick Soddy Work on chemistry of radioactive substances 1921 including the origin and nature of radioactive (chem) isotopes Francis Aston Discovery of isotopes in many non-radioactive 1922 elements, also enunciated the whole-number rule of (chem) atomic masses Charles Wilson Development of the cloud chamber for detecting 1927 charged particles Harold Urey Discovery of heavy hydrogen (deuterium) 1934 (chem) Frederic Joliot and Synthesis of several new radioactive elements 1935 Irene Joliot-Curie (chem) James Chadwick Discovery of the neutron 1935 Carl David Anderson Discovery of the positron 1936 Enrico Fermi New radioactive elements produced by neutron 1938 irradiation Ernest Lawrence -

Recollections and Reconstructions
@ ? Editor's Note: The Society's Nuclear Pioneer Citationfor 1977 goes not to an individual but to a distinguished group of individuals: those who took part in the epochal “ChicagoPile―experiment of Dec. 2, 1942, which produced thefirst successful atomicpile chain reaction. The 42 members ofthe Chicago Pilegroup are listed on page 591. One ofthose named, Harold M. Agnew, now Directorofthe Los Alamos Scient@flc Laboratory, is the Nuclear Pioneer Lecturerfor the 24th Annual Meeting. The guidingforce behind the Chicago Pile experiment was Enrico Fermi, recipient of the Nuclear Pioneer Citation of 1963. In the artick which follows, Laura Fermi recreates the excitement and mystery that surrounded the work ofher husband's research team in the early 1940s in Chicago. @. The FirstAtomic Pile: Recollections and Reconstructions by Laura Fermi @ . @ / - .-i@ ‘@:@ Enrico FermIat work, ca. 1942. As far as I can remember at the distance ofalmost 35 Perhaps not all husbands adhered as strictly as years, the first guests to arrive at our party on that Enrico to the rules of secrecy when talking to their beastly cold December night were the Zinns. Wally wives. He couldn't have been more tight-lipped. Once! and Jean. As they shook the snow off their coats, related some gossip to him: “Peoplesay that you stamping their feet on the floor, Wally turned to scientists at the Met Lab are seeking a cure for Enrico and said: “Congratulations!― I was surprised; cancer. .““Arewe?―he asked with his usual for Enrico had given me no hint that anything unusual imperturbable expression. -

The Endless Frontier 75Th Anniversary Edition
the endless frontier 75th Anniversary Edition VANNEVAR BUSH Reprinted in celebration of the National Science Foundation’s 70th anniversary1 | 1950-2020 Book cover photo: © Arnold Newman Collection via Getty Images SCIENCE THE ENDLESS FRONTIER A Report to the President by VANNEVAR BUSH Director of the Ofce of Scientifc Research and Development July 1945 Foreword by France A. Crdova, 14th Director of NSF Reissued by the National Science Foundation in celebration of the agency’s 70th anniversary and the 75th anniversary of Science—the Endless Frontier CELEBRATING NSF’S 70th BIRTHDAY By France A. Crdova, 14th Director of NSF “...basic research is the pacemaker of technological progress.” That statement is as relevant today as it was in 1945 when Vannevar Bush wrote it in his landmark tract, Science—the Endless Frontier. In the report, submitted to President Harry S. Truman, Bush made the case for creating a new agency that he and others felt was needed to support the underlying basic research essential for combatting disease, ensuring national security, and increasing the standard of living, including supporting new industries and jobs. Bush drew an important lesson from directing the Office of Scientific Research and Development during World War II: “The most important ways in which the Government can promote industrial research are to increase the flow of new scientific knowledge through support of basic research and to aid in the development of scientific talent.” Bush desired that the benefits of scientific re- search realized during the war could have even wider application postwar. He advocated for government funding of basic research in universities, colleges, and research institutes because that’s where the talent was. -

MIT-NSE by the 1930S It Was Time to Discover Fission. We Knew About Isoto
Prelude to the Manhattan Project Michael V. Hynes – MIT-NSE By the 1930s it was time to discover fission. We knew about isotopes. The Curies and Becquerel had discovered radioactivity. Rutherford had discovered the nucleus. Chadwick had discovered the neutron. Einstein had discovered special relativity and the famous equation E=mc2. Bethe was even publishing about fusion as the source of energy for the Sun. So, it was time. By the 1930s a human drama was unfolding in Europe and in the Pacific – the rise of fascism. The events of this drama engulfed the world in war and created a need on all sides for weaponry that could defeat the enemy by whatever means. The intersection of scientific inevitability and war created the environment for the advent of nuclear weapons. Science was practiced was very differently in the 1920s and 1930s than today. In that era science was highly specialized and compartmentalized. If you were a metallurgist for example it was unlikely that you would have anything to do with a physicist – that all changed in the1940s. In 1933 Leo Szilard (Fig. 1), a Hungarian physicist who took refuge in London from Nazi Germany, read a paper by Rutherford that ridiculed the idea of getting energy from nuclear transmutations. Szilard realized that if you could find an element which is split by neutrons and which would emit two neutrons in the process, then a chain reaction could be started. This is the basic idea of a nuclear weapon -- to generate energy from the chain reaction. Szilard was a chemist by training and knew about the idea of a chemical chain reaction. -

Otto Stern Annalen 4.11.11
(To be published by Annalen der Physik in December 2011) Otto Stern (1888-1969): The founding father of experimental atomic physics J. Peter Toennies,1 Horst Schmidt-Böcking,2 Bretislav Friedrich,3 Julian C.A. Lower2 1Max-Planck-Institut für Dynamik und Selbstorganisation Bunsenstrasse 10, 37073 Göttingen 2Institut für Kernphysik, Goethe Universität Frankfurt Max-von-Laue-Strasse 1, 60438 Frankfurt 3Fritz-Haber-Institut der Max-Planck-Gesellschaft Faradayweg 4-6, 14195 Berlin Keywords History of Science, Atomic Physics, Quantum Physics, Stern- Gerlach experiment, molecular beams, space quantization, magnetic dipole moments of nucleons, diffraction of matter waves, Nobel Prizes, University of Zurich, University of Frankfurt, University of Rostock, University of Hamburg, Carnegie Institute. We review the work and life of Otto Stern who developed the molecular beam technique and with its aid laid the foundations of experimental atomic physics. Among the key results of his research are: the experimental test of the Maxwell-Boltzmann distribution of molecular velocities (1920), experimental demonstration of space quantization of angular momentum (1922), diffraction of matter waves comprised of atoms and molecules by crystals (1931) and the determination of the magnetic dipole moments of the proton and deuteron (1933). 1 Introduction Short lists of the pioneers of quantum mechanics featured in textbooks and historical accounts alike typically include the names of Max Planck, Albert Einstein, Arnold Sommerfeld, Niels Bohr, Max von Laue, Werner Heisenberg, Erwin Schrödinger, Paul Dirac, Max Born, and Wolfgang Pauli on the theory side, and of Wilhelm Conrad Röntgen, Ernest Rutherford, Arthur Compton, and James Franck on the experimental side. However, the records in the Archive of the Nobel Foundation as well as scientific correspondence, oral-history accounts and scientometric evidence suggest that at least one more name should be added to the list: that of the “experimenting theorist” Otto Stern. -

Book Review Tuxedo Park, by Jennet Conant (Simon & Schuster, 2002), 330 Pp., ISBN 0684872870, $26.00
Book Review Tuxedo Park, by Jennet Conant (Simon & Schuster, 2002), 330 pp., ISBN 0684872870, $26.00 Reviewed by Jane A. Roman Department of Chemistry, Regis College, Weston, MA In the early chapters of this book, Jennet Conant, granddaughter of James Conant former President of Harvard and esteemed scientist, describes brilliantly the life of Alfred Lee Loomis, philanthropist, scientist and Wall Street tycoon. Prompted by the mysterious circumstances surrounding the suicide of her granduncle, William Richards, son of Nobel laureate Theodore William Richards, Jennet using her granduncles’ notes and letters, wrote this biography, which is a small but significant chapter in the history of American science. Her accounts of Loomis depict his relationships with many Nobel Laureates in science and also detail an illicit love affair Loomis had with Manette Hobart, the wife of Garrett A. Hobart III, his protégé and secretary at Tuxedo Park. The setting for most of the book is the region of Tuxedo Park in Orange County, New York where Loomis established a research laboratory, funded solely by his personal fortune. Very important discoveries in radar detection, atomic fission, and other wartime inventions that led the allies to victory over the Germans were made there. Because the circumstances surrounding her granduncle’s death and the hush-up carried out by her family were indicative of the gentry at that time, Ms. Conant was prompted to reveal in her novel the relationship her granduncle had with Loomis in Tuxedo Park. From his papers and letters, she describes how Richards and his friend at Princeton, George Kistiakowsky, came to work at Tuxedo Park, often referring to it as a private scientific playground in the Ramapo Mountains. -
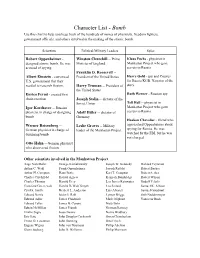
Character List
Character List - Bomb Use this chart to help you keep track of the hundreds of names of physicists, freedom fighters, government officials, and others involved in the making of the atomic bomb. Scientists Political/Military Leaders Spies Robert Oppenheimer - Winston Churchill -- Prime Klaus Fuchs - physicist in designed atomic bomb. He was Minister of England Manhattan Project who gave accused of spying. secrets to Russia Franklin D. Roosevelt -- Albert Einstein - convinced President of the United States Harry Gold - spy and Courier U.S. government that they for Russia KGB. Narrator of the needed to research fission. Harry Truman -- President of story the United States Enrico Fermi - created first Ruth Werner - Russian spy chain reaction Joseph Stalin -- dictator of the Tell Hall -- physicist in Soviet Union Igor Korchatov -- Russian Manhattan Project who gave physicist in charge of designing Adolf Hitler -- dictator of secrets to Russia bomb Germany Haakon Chevalier - friend who Werner Reisenberg -- Leslie Groves -- Military approached Oppenheimer about German physicist in charge of leader of the Manhattan Project spying for Russia. He was designing bomb watched by the FBI, but he was not charged. Otto Hahn -- German physicist who discovered fission Other scientists involved in the Manhattan Project: Aage Niels Bohr George Kistiakowsky Joseph W. Kennedy Richard Feynman Arthur C. Wahl Frank Oppenheimer Joseph Rotblat Robert Bacher Arthur H. Compton Hans Bethe Karl T. Compton Robert Serber Charles Critchfield Harold Agnew Kenneth Bainbridge Robert Wilson Charles Thomas Harold Urey Leo James Rainwater Rudolf Pelerls Crawford Greenewalt Harold DeWolf Smyth Leo Szilard Samuel K. Allison Cyril S. Smith Herbert L. Anderson Luis Alvarez Samuel Goudsmit Edward Norris Isidor I. -

Lise Meitner 1878 – 1968
Discoveries that changed the world: 1932 – 1942 James Chadwick 1891 – 1974 Lise Meitner 1878 – 1968 I „The road to the neutron“ Staff and research students at the Cavendish Laboratory, Cambridge, 1923. (Names from left to right. Front row: J. Chadwick, G. Stead, F.W. Aston, Prof. Sir J. J. Thomson, Prof. Sir E. Rutherford, J.A. Crowther, Miss B. Trevelyan, G.I. Taylor, Second row: P. Kapitza, H. de W. Smyth, T. Alty, J.E. Crackston, H. Robinson, L.F. Curtiss, E.S. Bieler, A.G.D. West, P. Mercier. Back row: P.M.S. Blackett, R.E. Clay, H.W.B. Skinner, H.D. Griffith, A.W. Barton, L.F. Bates, J.S. Rogers, K.G. Emeleus.) The room which Rutherford and Chadwick used for their scattering experiments in the 1920s. The work was carried out in the dark, often to the accompaniment of Rutherford singing „Onward Christian Soldiers“. Rutherford had already proposed the neutron in 1920 in his Bakerian Lecture at the Royal Society. He talked about a “neutral doublet” (at that time considered a proton and electron) that could be difficult to detect and move easily through matter. Curie & Joliot published (incorrectly) in Jan. 1932 the observation: 9Be + 4He → 12C + 1n I. Curie and F. Joliot, C. R. Acad. Sci. Paris 194, 273 (1932) When the radiation was passed through wax the ionisation increased! This increase was due to knock-on protons. To explain this the Curie’s suggested that the emission was of a 55 MeV γ ray, an energy much greater than anything yet seen! Moreover, the radiation also passed through lead This experiment was first performed in 1930 by Walter Bothe and Herbet Becker at U. -

Peaceful Berkelium
in your element Peaceful berkelium The first new element produced after the Second World War has led a rather peaceful life since entering the period table — until it became the target of those producing superheavy elements, as Andreas Trabesinger describes. f nomenclature were transitive, element 97 with the finding that in Bk(iii) compounds would be named after an Irish bishop spin–orbit coupling leads to a mixing of the Iwhose philosophical belief was that first excited state and the ground state. This material things do not exist. But between gives rise to unexpected electronic properties the immaterialist George Berkeley and the not present in analogous lanthanide element berkelium stands, of course, the structures containing terbium. Even more fine Californian city of Berkeley (pictured), recently5, a combined experimental and where the element was first produced in computational study revealed that berkelium December 1949. The city became the eponym can have stabilized +iii and +iV oxidation of element 97 “in a manner similar to that states also under mild aqueous conditions, used in naming its chemical homologue indicating a path to separating it from other terbium […] whose name was derived from lanthanides and actinides. the town of Ytterby, Sweden, where the rare The main use of berkelium, however — earth minerals were first found”1. arguably one that wouldn’t have met with A great honour for the city? The mayor Bishop Berkeley’s approval — remains a of Berkeley at the time reportedly displayed PHOTO STOCK / ALAMY AF ARCHIVE distinctly material one: as the target for “a complete lack of interest when he was the synthesis of other transuranium and called with the glad tidings”2.