Aug. 21, 1973 3,753,920
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Assessment of Portable HAZMAT Sensors for First Responders
The author(s) shown below used Federal funds provided by the U.S. Department of Justice and prepared the following final report: Document Title: Assessment of Portable HAZMAT Sensors for First Responders Author(s): Chad Huffman, Ph.D., Lars Ericson, Ph.D. Document No.: 246708 Date Received: May 2014 Award Number: 2010-IJ-CX-K024 This report has not been published by the U.S. Department of Justice. To provide better customer service, NCJRS has made this Federally- funded grant report available electronically. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. Department of Justice. Assessment of Portable HAZMAT Sensors for First Responders DOJ Office of Justice Programs National Institute of Justice Sensor, Surveillance, and Biometric Technologies (SSBT) Center of Excellence (CoE) March 1, 2012 Submitted by ManTech Advanced Systems International 1000 Technology Drive, Suite 3310 Fairmont, West Virginia 26554 Telephone: (304) 368-4120 Fax: (304) 366-8096 Dr. Chad Huffman, Senior Scientist Dr. Lars Ericson, Director UNCLASSIFIED This project was supported by Award No. 2010-IJ-CX-K024, awarded by the National Institute of Justice, Office of Justice Programs, U.S. Department of Justice. The opinions, findings, and conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect those of the Department of Justice. This document is a research report submitted to the U.S. Department of Justice. This report has not been published by the Department. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. -

Chemical Name Federal P Code CAS Registry Number Acutely
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extremely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extremely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extremely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extremely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extremely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extremely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extremely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extremely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extremely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

Acutely / Extremely Hazardous Waste List
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extemely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extemely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extemely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extemely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extemely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extemely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extemely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extemely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extemely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extemely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extemely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extemely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extemely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

Proceedings of the Nuclear Energy Agency International Workshop on Chemical Hazards in Fuel Cycle Facilities Nuclear Processing
Nuclear Safety NEA/ CSNI/R(2019)9/ADD1 May 2019 www.oecd-nea.org Proceedings of the Nuclear Energy Agency International Workshop on Chemical Hazards in Fuel Cycle Facilities Nuclear Processing Appendix C NEA/CSNI/R(2019)9/ADD1 The non-radiological risks involving dangerous chemicals in nuclear fuel cycle facilities - French framework regulation - Youcef HEMIMOU, Brice DELIME, Raffaello AMOROSI, Josquin VERNON French Nuclear Safety Authority 15, rue Louis Lejeune CS70013 – 92541 Montrouge Cedex [email protected] Accidents involving hazardous chemicals pose a significant threat to the population and the environment. Among nuclear facilities, this threat applies in particular to the fuel cycle facilities. As a consequence, the activities related to hazardous chemicals are covered by a legal framework that, depending on the nature of the activity and the associated risks, aims to guarantee that, they will not be likely to be detrimental to safety, public health and environment [1]. The aim of this article is to present a summary of the main regulations involving dangerous chemicals in nuclear fuel cycle facilities that have to be taken into account in the safety demonstration. The latter must prove that the risks of an accident - radiological or not - and the scale of its consequences, given the current state of knowledge, practices and the vulnerability of the installation environment, are as low as possible under acceptable economic conditions. Key words : nuclear fuel cycle facilities, safety demonstration, principle of defence in depth, deterministic approach, probabilistic approach, dangerous chemicals, non-radiological risks, Seveso Directive, domino effects. 1. THE DANGEROUS SUBSTANCES IN NUCLEAR FUEL CYCLE FACILITIES 1.1. -

United States Patent Office Patented Mar
3,373,000 United States Patent Office Patented Mar. 2, 1968 2 3,373,000 monofluoride is also an economical reactant, since it is PROCESS FOR PREPARING TETRAFLUORDES conveniently prepared by the disproportionation of ele AND HEXAFLUOR DES mental chlorine in liquid hydrogen fluoride, thereby avoid James J. Pitts, West Haven, and Albert W. Jache, North ing the use of expensive elemental fluorine. The selective Haven, Conn., assignors to Olin Mathieson Chemical fluorination process of this invention is particularly Sur Corporation, a corporation of Virginia prising and unexpected in view of the prior art which No Drawing. Filed Nov. 1, 1966, Ser. No. 591,079 teaches that chlorine monofluoride adds to a wide variety 9 Claims. (Cl. 23-352) of compounds, thereby acting as a chloro-fluorinating agent. For instance, U.S. Patent 3,035,893 discloses the 10 preparation of sulfur chloride pentafluoride from Sulfur ABSTRACT OF THE DISCLOSURE tetrafluoride and chlorine monofluoride. The tetrafluorides and hexafluorides of sulfur, selenium According to the process of this invention, the tetra and tellurium are provided by reacting chlorine mono fluorides and hexafluorides of sulfur, selenium and tel fluoride with sulfur, selenium, tellurium, metal sulfides, lurium are provided by reacting chlorine monofluoride metal selenides or metal tellurides. The hexafluorides of 15 with a material selected from the group consisting of Sul tungsten, molybdenum and uranium are provided by the fur, selenium, tellurium, metal sulfides, metal selenides reaction of chlorine monofluoride with the metals, their and metal tellurides. The hexafluorides of tungsten, oxides, sulfides and salts containing the metallate anion. molybdenum and uranium are provided by reacting These tetrafluorides and hexafluorides are well-known chlorine monofluoride with a material selected from the compounds, useful as fluorinating agents, gaseous di 20 group consisting of tungsten; molybdenum, uranium; the electrics, sources of high purity metallic powders, etc. -

List of Lists
United States Office of Solid Waste EPA 550-B-10-001 Environmental Protection and Emergency Response May 2010 Agency www.epa.gov/emergencies LIST OF LISTS Consolidated List of Chemicals Subject to the Emergency Planning and Community Right- To-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act • EPCRA Section 302 Extremely Hazardous Substances • CERCLA Hazardous Substances • EPCRA Section 313 Toxic Chemicals • CAA 112(r) Regulated Chemicals For Accidental Release Prevention Office of Emergency Management This page intentionally left blank. TABLE OF CONTENTS Page Introduction................................................................................................................................................ i List of Lists – Conslidated List of Chemicals (by CAS #) Subject to the Emergency Planning and Community Right-to-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act ................................................. 1 Appendix A: Alphabetical Listing of Consolidated List ..................................................................... A-1 Appendix B: Radionuclides Listed Under CERCLA .......................................................................... B-1 Appendix C: RCRA Waste Streams and Unlisted Hazardous Wastes................................................ C-1 This page intentionally left blank. LIST OF LISTS Consolidated List of Chemicals -

2020 Emergency Response Guidebook
2020 A guidebook intended for use by first responders A guidebook intended for use by first responders during the initial phase of a transportation incident during the initial phase of a transportation incident involving hazardous materials/dangerous goods involving hazardous materials/dangerous goods EMERGENCY RESPONSE GUIDEBOOK THIS DOCUMENT SHOULD NOT BE USED TO DETERMINE COMPLIANCE WITH THE HAZARDOUS MATERIALS/ DANGEROUS GOODS REGULATIONS OR 2020 TO CREATE WORKER SAFETY DOCUMENTS EMERGENCY RESPONSE FOR SPECIFIC CHEMICALS GUIDEBOOK NOT FOR SALE This document is intended for distribution free of charge to Public Safety Organizations by the US Department of Transportation and Transport Canada. This copy may not be resold by commercial distributors. https://www.phmsa.dot.gov/hazmat https://www.tc.gc.ca/TDG http://www.sct.gob.mx SHIPPING PAPERS (DOCUMENTS) 24-HOUR EMERGENCY RESPONSE TELEPHONE NUMBERS For the purpose of this guidebook, shipping documents and shipping papers are synonymous. CANADA Shipping papers provide vital information regarding the hazardous materials/dangerous goods to 1. CANUTEC initiate protective actions. A consolidated version of the information found on shipping papers may 1-888-CANUTEC (226-8832) or 613-996-6666 * be found as follows: *666 (STAR 666) cellular (in Canada only) • Road – kept in the cab of a motor vehicle • Rail – kept in possession of a crew member UNITED STATES • Aviation – kept in possession of the pilot or aircraft employees • Marine – kept in a holder on the bridge of a vessel 1. CHEMTREC 1-800-424-9300 Information provided: (in the U.S., Canada and the U.S. Virgin Islands) • 4-digit identification number, UN or NA (go to yellow pages) For calls originating elsewhere: 703-527-3887 * • Proper shipping name (go to blue pages) • Hazard class or division number of material 2. -

Ucri, 100554 Preprint
UCRI, 100554 PREPRINT x CHEMICAL THERMODYNAMICS OF TECHNETIUM 0 r0 0 0m 0 Joseph A.' Rard z C w1 m This paper was prepared for inclusion in the book CHEMICAL THERMODYNAMICS OF TECHNETIUM (to' be published by the Nuclear Energy Agency, Paris, France) February 1989 This is a preprint of a paper intended for publication in a journal or proceedings. Since changes may be made before publication, this preprint is made available with the understanding that it will not be cited or reproduced without the permission of the author. - DISCILAIMER This document was prepared as an account of work sponsored by asnagency of the United States Government Neither the United States Government nor the University of California nor any of their employees. makes any warranty, express or implieid, or assumes any legal liability or responsibility for the accuracy completeness, or useful- ness of any Information, apparatus. product, or process disclosed. or represents that Its use would not Iafringe privately owned rights. Reference hereln to any specific commercial products, process, or service by trade name. trademark. manufacturer, or otherwise. does not necessarily constitute or Imply its endorsement. recommendation, or favoring by the United States Government or the University of California. The views and opinions of authors expressed hereb do not necessarily state or reflect those of the United States Government or the University of California, and shall not be used for advertising or product endorsement purposes. -W This manuscript by JA Rard is to be part of a book entitled "CHEMICAL THERMODYNAMICS OF TECHNETIUM". It will be published by the Nuclear Energy Agency Data Bank at Saclay, France. -

Reducing Surface Recombination Velocity in Thin Perovskite Films
Thermochemistry of Ruthenium in Molten Fluoride Salts Metal or Melt? by Laurens G. Braskamp in partial fulfillment of the requirements for the degree of Bachelor of Science in Molecular Science and Technology at the Delft University of Technology to be defended publicly on Monday, August 20, 2018 at 14:30 Student numbers: 4411064 (TU Delft) 1517988 (Universiteit Leiden) Thesis Committee: dr. Elisa Capelli (supervisor) TU Delft dr. Anna L. Smith prof. dr. Rudy J.M. Konings Abstract The Molten Salt Reactor (MSR) is a member of safer and more sustainable fourth generation of nuclear reactors, the development of the might be crucial to continually secure supply of electrical energy from the near future onwards. Because the fluorides of ruthenium (44Ru) are a poorly investigated group of fission products that might be born during MSR operation, some computational research was done on their thermochemistry. Because little experimental data on these species has been obtained, the gas-phase molecular geometries and formation enthalpies of all known Ru-F binary compounds, RuF, RuF2, RuF3, RuF4, RuF5, RuF6, (RuF5)2 and (RuF5)3 are calculated by Density Functional Theory. The DFT/B3LYP method was employed with quasi-relativistic ECP28MWB pseudopotentials and basis set on ruthenium and cc-pVTZ basis set on fluorine. Standard molar entropies and heat capacities were then also calculated by statistical mechanics computations. From the obtained thermochemical data, part of which had to be optimized, and additional data on the solid and liquid phases, the Ru-F subsystem was assessed using the CALPHAD methodology. Based on the developed model, a phase diagram, Ellingham diagram and gas-phase equilibrium diagram were calculated. -
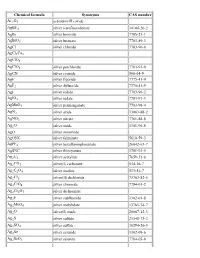
List of Chemical Formulas
Chemical formula Synonyms CAS number Ac2O3 actinium(III) oxide AgBF4 silver tetrafluoroborate 14104-20-2 AgBr silver bromide 7785-23-1 AgBrO3 silver bromate 7783-89-3 AgCl silver chloride 7783-90-6 AgCl3Cu2 AgClO3 AgClO4 silver perchlorate 7783-93-9 AgCN silver cyanide 506-64-9 AgF silver fluoride 7775-41-9 AgF2 silver difluoride 7775-41-9 AgI silver iodide 7783-96-2 AgIO3 silver iodate 7783-97-3 AgMnO4 silver permanganate 7783-98-4 AgN3 silver azide 13863-88-2 AgNO3 silver nitrate 7761-88-8 Ag2O silver oxide 1301-96-8 AgO silver monoxide AgONC silver fulminate 5610-59-3 AgPF6 silver hexafluorophosphate 26042-63-7 AgSNC silver thiocyanate 1701-93-5 Ag2C2 silver acetylide 7659-31-6 Ag2CO3 silver(I) carbonate 534-16-7 Ag2C2O4 silver oxalate 533-51-7 Ag2Cl2 silver(II) dichloride 75763-82-5 Ag2CrO4 silver chromate 7784-01-2 Ag2Cr2O7 silver dichromate Ag2F silver subfluoride 1302-01-8 Ag2MoO4 silver molybdate 13765-74-7 Ag2O silver(I) oxide 20667-12-3 Ag2S silver sulfide 21548-73-2 Ag2SO4 silver sulfate 10294-26-5 Ag2Se silver selenide 1302-09-6 Ag2SeO3 silver selenite 7784-05-6 Ag2SeO4 silver selenate 7784-07-8 Ag2Te silver(I) telluride 12002-99-2 Ag3Br2 silver dibromide 11078-32-3 Ag3Br3 silver tribromide 11078-33-4 Ag3Cl3 silver(III) trichloride 12444-96-1 Ag3I3 silver(III) triiodide 37375-12-5 Ag3PO4 silver phosphate 7784-09-0 AlBO aluminium boron oxide 12041-48-4 AlBO2 aluminium borate 61279-70-7 AlBr aluminium monobromide 22359-97-3 AlBr3 aluminium tribromide 7727-15-3 AlCl aluminium monochloride 13595-81-8 AlClF aluminium chloride fluoride -

CONTRIBUTORS to THIS VOLUME B. J. Aylett William A. Frad K. V. Kotegov 0.N
CONTRIBUTORS TO THIS VOLUME B. J. Aylett William A. Frad K. V. Kotegov 0.N. Pavlov H. L. Roberts V. P. Shvedov B. J. Wakefield J. F. Young Advances in INORGANIC CHEMISTRY AND RAD IOCH EM I STRY EDlTORS H. J. EMELEUS A. G. SHARPE University Chemical Laboratory Cambridge, England VOLUME /I I968 ACADEMIC PRESS New York and London COPYRIUHT 0 1968, BY ACADEMICPRESS, INC. ALL RIGHTS RESERVED. NO PART OF THIS BOOK MAY BE REPRODUCED IN ANY FORM, BY PHOTOSTAT, MICROFILM, OR ANY OTHER MEANS, WITHOUT WRITTEN PERMISSION FROM TEE PUBLISHERS. ACADEMIC PRESS, INC. 111 Fifth Avenue, New York, New York 10003 United Kingdom Edition published by ACADEMIC PRESS INC. (LONDON) LTD. Berkeley Square House, London W.l LIBRARYOF CONGRESSCATALOG CARD NVMBER:59-7692 PRINTED IN THE UNITED STATES OF AMERICA LIST OF CONTRIBUTORS Numbers in parentheses indicate the pages on which the authors’ contributions begin. B. J. AYLETT(249), Chemistry Department, Westjield College (University Of London), Hampstead, London, England WILLIAMA. FRAD(153), University of Missouri at Rolla, Rolla, Missouri K. V. KOTEGOV(I), Atomizdat, Moscow, U.S.S.R. 0. N. PAVLOV(I), Atomizdat, Moscow, U.S.S.R. H. L. ROBERTS(309), Imperial Chemical Industries Limited, Mond Division, Runcorn, Cheshire, England V. P. SHVEDOV(l), Atomizdat, Moscow, U.S.S.R. B. J. WAKEFIELD(341), Department of Chemistry and Applied Chemistry, University of Salford, Lancashire, England. J. F. YOUNG(91), Chemistry Division, D.S.I.R., Wellington, New Zealand V TECHNETIUM K. V. Kotegov, 0. N. Pavlov and V. P. Shvedov * Atomizdat, Moscow, U.S.S.R., Introduction .