Distinct Triterpene Synthases in the Laticifers of Euphorbia Lathyris
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ATP-Citrate Lyase Has an Essential Role in Cytosolic Acetyl-Coa Production in Arabidopsis Beth Leann Fatland Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 2002 ATP-citrate lyase has an essential role in cytosolic acetyl-CoA production in Arabidopsis Beth LeAnn Fatland Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Molecular Biology Commons, and the Plant Sciences Commons Recommended Citation Fatland, Beth LeAnn, "ATP-citrate lyase has an essential role in cytosolic acetyl-CoA production in Arabidopsis " (2002). Retrospective Theses and Dissertations. 1218. https://lib.dr.iastate.edu/rtd/1218 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. ATP-citrate lyase has an essential role in cytosolic acetyl-CoA production in Arabidopsis by Beth LeAnn Fatland A dissertation submitted to the graduate faculty in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Major: Plant Physiology Program of Study Committee: Eve Syrkin Wurtele (Major Professor) James Colbert Harry Homer Basil Nikolau Martin Spalding Iowa State University Ames, Iowa 2002 UMI Number: 3158393 INFORMATION TO USERS The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleed-through, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. -

Characterization of the Ergosterol Biosynthesis Pathway in Ceratocystidaceae
Journal of Fungi Article Characterization of the Ergosterol Biosynthesis Pathway in Ceratocystidaceae Mohammad Sayari 1,2,*, Magrieta A. van der Nest 1,3, Emma T. Steenkamp 1, Saleh Rahimlou 4 , Almuth Hammerbacher 1 and Brenda D. Wingfield 1 1 Department of Biochemistry, Genetics and Microbiology, Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria 0002, South Africa; [email protected] (M.A.v.d.N.); [email protected] (E.T.S.); [email protected] (A.H.); brenda.wingfi[email protected] (B.D.W.) 2 Department of Plant Science, University of Manitoba, 222 Agriculture Building, Winnipeg, MB R3T 2N2, Canada 3 Biotechnology Platform, Agricultural Research Council (ARC), Onderstepoort Campus, Pretoria 0110, South Africa 4 Department of Mycology and Microbiology, University of Tartu, 14A Ravila, 50411 Tartu, Estonia; [email protected] * Correspondence: [email protected]; Fax: +1-204-474-7528 Abstract: Terpenes represent the biggest group of natural compounds on earth. This large class of organic hydrocarbons is distributed among all cellular organisms, including fungi. The different classes of terpenes produced by fungi are mono, sesqui, di- and triterpenes, although triterpene ergosterol is the main sterol identified in cell membranes of these organisms. The availability of genomic data from members in the Ceratocystidaceae enabled the detection and characterization of the genes encoding the enzymes in the mevalonate and ergosterol biosynthetic pathways. Using Citation: Sayari, M.; van der Nest, a bioinformatics approach, fungal orthologs of sterol biosynthesis genes in nine different species M.A.; Steenkamp, E.T.; Rahimlou, S.; of the Ceratocystidaceae were identified. -

Plant-Specific Glutaredoxin ROXY9 Regulates Hyponastic Growth by Inhibiting
Plant-specific glutaredoxin ROXY9 regulates hyponastic growth by inhibiting TGA1 function Dissertation for the award of the degree “Doctor of Philosophy” (Ph.D.) Division of Mathematics and Natural Sciences of the Georg-August-Universität Göttingen within the doctoral program Biology of the Georg-August University School of Science (GAUSS) Submitted by Ning Li from Shandong, China Göttingen 2017 Thesis Committee Prof. Dr. Christiane Gatz (Department of Plant Molecular Biology and Physiology) Prof. Dr. Volker Lipka (Department of Plant Cell Biology) Dr. Corinna Thurow (Department of Plant Molecular Biology and Physiology) Members of the Examination Board Reviewer: Prof. Dr. Christiane Gatz (Department of Plant Molecular Biology and Physiology) Second reviewer: Prof. Dr. Volker Lipka (Department of Plant Cell Biology) Further members of the Examination Board: Prof. Dr. Ivo Feussner (Department of Plant Biochemistry) PD Dr. Thomas Teichmann (Department of Plant Cell Biology) Dr. Marcel Wiermer (Department of Plant Cell Biology) PD. Dr. Martin Fulda (Department of Plant Biochemistry) Date of the oral examination: 30.03.2017 1 Introduction ................................................................................................................ 1 1.1 Hyponastic growth ............................................................................................ 1 1.1.1 Ethylene and hyponastic growth in Arabidopsis thaliana ...................... 2 1.1.2 Photoreceptors and hyponastic growth ................................................ -

Aquatic Toxicology 176 (2016) 116–127
Aquatic Toxicology 176 (2016) 116–127 Contents lists available at ScienceDirect Aquatic Toxicology j ournal homepage: www.elsevier.com/locate/aquatox Linking the response of endocrine regulated genes to adverse effects on sex differentiation improves comprehension of aromatase inhibition in a Fish Sexual Development Test a,∗ b b b Elke Muth-Köhne , Kathi Westphal-Settele , Jasmin Brückner , Sabine Konradi , c a a,1 d,1 Viktoria Schiller , Christoph Schäfers , Matthias Teigeler , Martina Fenske a Fraunhofer IME, Department of Ecotoxicology, Auf dem Aberg 1, 57392 Schmallenberg, Germany b German Environment Agency (UBA), Woerlitzer Platz 1, 06844 Dessau, Germany c Fraunhofer IME, Attract Group UNIFISH, Forckenbeckstraße 6, 52074 Aachen, Germany d Fraunhofer IME, Project Group Translational Medicine and Pharmacology TMP, Forckenbeckstraße 6, 52074 Aachen, Germany a r a t i c l e i n f o b s t r a c t Article history: The Fish Sexual Development Test (FSDT) is a non-reproductive test to assess adverse effects of endocrine Received 23 December 2015 disrupting chemicals. With the present study it was intended to evaluate whether gene expression end- Received in revised form 13 April 2016 points would serve as predictive markers of endocrine disruption in a FSDT. For proof-of-concept, a FSDT Accepted 19 April 2016 according to the OECD TG 234 was conducted with the non-steroidal aromatase inhibitor fadrozole (test Available online 20 April 2016 concentrations: 10 g/L, 32 g/L, 100 g/L) using zebrafish (Danio rerio). Gene expression analyses using quantitative RT-PCR were included at 48 h, 96 h, 28 days and 63 days post fertilization (hpf, dpf). -

Friedelin Synthase from Maytenus Ilicifolia
www.nature.com/scientificreports OPEN Friedelin Synthase from Maytenus ilicifolia: Leucine 482 Plays an Essential Role in the Production of Received: 09 May 2016 Accepted: 20 October 2016 the Most Rearranged Pentacyclic Published: 22 November 2016 Triterpene Tatiana M. Souza-Moreira1, Thaís B. Alves1, Karina A. Pinheiro1, Lidiane G. Felippe1, Gustavo M. A. De Lima2, Tatiana F. Watanabe1, Cristina C. Barbosa3, Vânia A. F. F. M. Santos1, Norberto P. Lopes4, Sandro R. Valentini3, Rafael V. C. Guido2, Maysa Furlan1 & Cleslei F. Zanelli3 Among the biologically active triterpenes, friedelin has the most-rearranged structure produced by the oxidosqualene cyclases and is the only one containing a cetonic group. In this study, we cloned and functionally characterized friedelin synthase and one cycloartenol synthase from Maytenus ilicifolia (Celastraceae). The complete coding sequences of these 2 genes were cloned from leaf mRNA, and their functions were characterized by heterologous expression in yeast. The cycloartenol synthase sequence is very similar to other known OSCs of this type (approximately 80% identity), although the M. ilicifolia friedelin synthase amino acid sequence is more related to β-amyrin synthases (65–74% identity), which is similar to the friedelin synthase cloned from Kalanchoe daigremontiana. Multiple sequence alignments demonstrated the presence of a leucine residue two positions upstream of the friedelin synthase Asp-Cys-Thr-Ala-Glu (DCTAE) active site motif, while the vast majority of OSCs identified so far have a valine or isoleucine residue at the same position. The substitution of the leucine residue with valine, threonine or isoleucine in M. ilicifolia friedelin synthase interfered with substrate recognition and lead to the production of different pentacyclic triterpenes. -

Biocatalysis in the Chemistry of Lupane Triterpenoids
molecules Review Biocatalysis in the Chemistry of Lupane Triterpenoids Jan Bachoˇrík 1 and Milan Urban 2,* 1 Department of Organic Chemistry, Faculty of Science, Palacký University in Olomouc, 17. listopadu 12, 771 46 Olomouc, Czech Republic; [email protected] 2 Medicinal Chemistry, Faculty of Medicine and Dentistry, Institute of Molecular and Translational Medicine, Palacký University in Olomouc, Hnˇevotínská 5, 779 00 Olomouc, Czech Republic * Correspondence: [email protected] Abstract: Pentacyclic triterpenes are important representatives of natural products that exhibit a wide variety of biological activities. These activities suggest that these compounds may represent potential medicines for the treatment of cancer and viral, bacterial, or protozoal infections. Naturally occurring triterpenes usually have several drawbacks, such as limited activity and insufficient solubility and bioavailability; therefore, they need to be modified to obtain compounds suitable for drug development. Modifications can be achieved either by methods of standard organic synthesis or with the use of biocatalysts, such as enzymes or enzyme systems within living organisms. In most cases, these modifications result in the preparation of esters, amides, saponins, or sugar conjugates. Notably, while standard organic synthesis has been heavily used and developed, the use of the latter methodology has been rather limited, but it appears that biocatalysis has recently sparked considerably wider interest within the scientific community. Among triterpenes, derivatives of lupane play important roles. This review therefore summarizes the natural occurrence and sources of lupane triterpenoids, their biosynthesis, and semisynthetic methods that may be used for the production of betulinic acid from abundant and inexpensive betulin. Most importantly, this article compares chemical transformations of lupane triterpenoids with analogous reactions performed by Citation: Bachoˇrík,J.; Urban, M. -

Steroidal Triterpenes of Cholesterol Synthesis
Molecules 2013, 18, 4002-4017; doi:10.3390/molecules18044002 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Review Steroidal Triterpenes of Cholesterol Synthesis Jure Ačimovič and Damjana Rozman * Centre for Functional Genomics and Bio-Chips, Faculty of Medicine, Institute of Biochemistry, University of Ljubljana, Zaloška 4, Ljubljana SI-1000, Slovenia; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +386-1-543-7591; Fax: +386-1-543-7588. Received: 18 February 2013; in revised form: 19 March 2013 / Accepted: 27 March 2013 / Published: 4 April 2013 Abstract: Cholesterol synthesis is a ubiquitous and housekeeping metabolic pathway that leads to cholesterol, an essential structural component of mammalian cell membranes, required for proper membrane permeability and fluidity. The last part of the pathway involves steroidal triterpenes with cholestane ring structures. It starts by conversion of acyclic squalene into lanosterol, the first sterol intermediate of the pathway, followed by production of 20 structurally very similar steroidal triterpene molecules in over 11 complex enzyme reactions. Due to the structural similarities of sterol intermediates and the broad substrate specificity of the enzymes involved (especially sterol-Δ24-reductase; DHCR24) the exact sequence of the reactions between lanosterol and cholesterol remains undefined. This article reviews all hitherto known structures of post-squalene steroidal triterpenes of cholesterol synthesis, their biological roles and the enzymes responsible for their synthesis. Furthermore, it summarises kinetic parameters of enzymes (Vmax and Km) and sterol intermediate concentrations from various tissues. Due to the complexity of the post-squalene cholesterol synthesis pathway, future studies will require a comprehensive meta-analysis of the pathway to elucidate the exact reaction sequence in different tissues, physiological or disease conditions. -

Expression of Selected Genes Involved in Steroidogenesis in the Course of Enucleation-Induced Rat Adrenal Regeneration
INTERNATIONAL JOURNAL OF MOLECULAR MEDICINE 33: 613-623, 2014 Expression of selected genes involved in steroidogenesis in the course of enucleation-induced rat adrenal regeneration MARIANNA TYCZEWSKA, MARCIN RUCINSKI, AGNIESZKA ZIOLKOWSKA, MARCIN TREJTER, MARTA SZYSZKA and LUDWIK K. MALENDOWICZ Department of Histology and Embryology, Poznan University of Medical Sciences, Poznan, Poland Received October 28, 2013; Accepted December 6, 2013 DOI: 10.3892/ijmm.2013.1599 Abstract. The enucleation-induced (EI) rapid proliferation however, throughout the entire experimental period, there were of adrenocortical cells is followed by their differentiation, no statistically significant differences observed. After the initial the degree of which may be characterized by the expression decrease in steroidogenic factor 1 (Sf-1) mRNA levels observed of genes directly and indirectly involved in steroid hormone on the 1st day of the experiment, a marked upregulation in its biosynthesis. In this study, out of 30,000 transcripts of genes expression was observed from there on. Data from the current identified by means of Affymetrix Rat Gene 1.1 ST Array, we study strongly suggest the role of Fabp6, Lipe and Soat1 in aimed to select genes (either up- or downregulated) involved supplying substrates of regenerating adrenocortical cells for in steroidogenesis in the course of enucleation-induced adrenal steroid synthesis. Our results indicate that during the first days regeneration. On day 1, we found 32 genes with altered of adrenal regeneration, intense synthesis of cholesterol may expression levels, 15 were upregulated and 17 were down- occur, which is then followed by its conversion into cholesteryl regulated [i.e., 3β-hydroxysteroid dehydrogenase (Hsd3β), esters. -
Figures Intermediary Ontogeny.Pptx
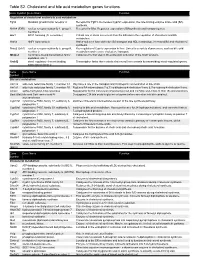
Table S2. Cholesterol and bile acid metabolism genes functions. Gene Symbol Gene Name Function Regulation of cholesterol and/or bile acid metabolism Fgfr4 fibroblast growth factor receptor 4 Receptor for Fgf15. Decreases Cyp7a1 expression, the rate-limiting enzyme in bile acid (BA) synthesis. Nr1h4 (FXR) nuclear receptor subfamily 1, group H, Receptor for BAs. Regulates expression of BAsynthesis and transport genes. member 4 Arv1 ARV1 homolog (S. cerevisiae) Critical role in sterol movement from the ER and in the regulation of cholesterol and BA metabolism. Hnf1a HNF1 homeobox A Hnf1a-null mice have defective BA transport and HDL metabolism, increased BA and cholesterol synthesis. Nr5a2 (Lrh1) nuclear receptor subfamily 5, group A, Key regulator of Cyp7a expression in liver. Linked to a variety of processes, such as bile acid member 2 metabolism and reverse cholesterol transport. Mbtps1 membrane-bound transcription factor Catalyzes the first step in the proteolytic activation of the Srebf proteins. peptidase, site 1 Srebf2 sterol regulatory element binding Transcription factor that controls cholesterol homeostasis by transcribing sterol-regulated genes. transcription factor 2 Gene Gene Name Function Symbol Bile acid metabolism Akr1c6 aldo-keto reductase family 1, member C1 May have a role in the transport and intrahepatic concentration of bile acids. Akr1d1 aldo-keto reductase family 1, member D1 Reduces BA intermediates 7-α,12-α-dihydroxy-4-cholesten-3-one & 7-α-hydroxy-4-cholesten-3-one. Amacr alpha-methylacyl-CoA racemase Responsible for the conversion of pristanoyl-CoA and C27-bile acyl-CoAs to their (S)-stereoisomers. Baat (Bat) bile acid CoA: amino acid N- Conjugates C24 bile acids to glycine or taurine before excretion into bile canaliculi. -

生物合成薯蓣皂素的途径设计及关键酶分析 Chinese Journal of Biotechnology Apr
1178 生物工程学报 孙忠义 等/生物合成薯蓣皂素的途径设计及关键酶分析 Chinese Journal of Biotechnology http://journals.im.ac.cn/cjbcn Apr. 25, 2021, 37(4): 1178−1188 DOI: 10.13345/j.cjb.200389 ©2021 Chin J Biotech, All rights reserved ·综 述· 生物合成薯蓣皂素的途径设计及关键酶分析 1 1 2 1 孙忠义 ,赵鹏 ,葛喜珍 ,田平芳 1 北京化工大学 生命科学与技术学院,北京 100029 2 北京联合大学 生物化学工程学院,北京 100023 孙忠义, 赵鹏, 葛喜珍, 等. 生物合成薯蓣皂素的途径设计及关键酶分析. 生物工程学报, 2021, 37(4): 1178-1188. Sun ZY, Zhao P, Ge XZ, et al. Pathway design and key enzyme analysis of diosgenin biosynthesis. Chin J Biotech, 2021, 37(4): 1178–1188. 摘 要: 薯蓣皂素是一种天然甾体皂苷元,可作为数百种类固醇药物的前体,具有重要药用价值。目前工业生产 薯蓣皂素主要依赖化学提取法,因此该法依赖植物材料和耕地且对环境有害。随着代谢工程和合成生物学的发展, 生物合成法受到广泛关注。文中综述了生物合成薯蓣皂素的代谢途径和关键酶,并在酿酒酵母 Saccharomyces cerevisiae 中设计其异源合成途径,提出改造策略,以期为全生物合成薯蓣皂素提供有价值的参考。 关键词: 薯蓣皂素,生物合成途径,关键酶,异源表达 Pathway design and key enzyme analysis of diosgenin biosynthesis Zhongyi Sun1, Peng Zhao1, Xizhen Ge2, and Pingfang Tian1 1 College of Life Science and Technology, Beijing University of Chemical Technology, Beijing 100029, China 2 College of Biochemical Engineering, Beijing Union University, Beijing 100023, China Abstract: As a naturally occurring steroid sapogenin, diosgenin acts as the precursor of hundreds of steroid medicines, and thereby has important medicinal value. Currently, industrial production of diosgenin relies primarily on chemical extraction from plant materials. Clearly, this strategy shows drawbacks of excessive reliance on plant materials and farmland as well as environment pollution. Due to development of metabolic engineering and synthetic biology, bio-production of diosgenin has garnered plenty of attention. Although the biosynthetic pathways of diosgenin have not been completely identified, in this review, we outline the identified biosynthetic pathways and key enzymes. In particular, we suggest heterologous biosynthesis of diosgenin in Saccharomyces cerevisiae. -

The Triforc Database: a Comprehensive Up-To-Date Resource
D586–D594 Nucleic Acids Research, 2018, Vol. 46, Database issue Published online 17 October 2017 doi: 10.1093/nar/gkx925 The TriForC database: a comprehensive up-to-date resource of plant triterpene biosynthesis Karel Miettinen1,2, Sabrina Inigo˜ 1,2, Lukasz Kreft3, Jacob Pollier1,2,ChristofDeBo3, Alexander Botzki3, Frederik Coppens1,2, Søren Bak4 and Alain Goossens1,2,* 1Ghent University, Department of Plant Biotechnology and Bioinformatics, 9052 Ghent, Belgium, 2VIB Center for Plant Systems Biology, 9052 Ghent, Belgium, 3VIB Bioinformatics Core, 9052 Ghent, Belgium and 4Plant Biochemistry Laboratory and Villum Research Center ‘Plant Plasticity’, Copenhagen Plant Science Center, Department of Plant and Environmental Sciences, University of Copenhagen, Copenhagen, Denmark Received August 14, 2017; Revised September 23, 2017; Editorial Decision September 26, 2017; Accepted October 03, 2017 ABSTRACT INTRODUCTION Triterpenes constitute a large and important class of Importance of triterpenes plant natural products with diverse structures and Triterpenes compose a diverse class of plant natural prod- functions. Their biological roles range from mem- ucts, both in structure and function. They comprise (i) pri- brane structural components over plant hormones mary metabolites such as the phytosterols, which are the to specialized plant defence compounds. Further- indispensable structural components of cell membranes, more, triterpenes have great potential for a vari- and hormones, such as brassinosteroids, and (ii) specialized ety of commercial applications such as vaccine ad- (also called secondary) metabolites with diverse biological juvants, anti-cancer drugs, food supplements and functions (1,2). The latter include among others defence agronomic agents. Their biosynthesis is carried out compounds with antimicrobial, antifungal, antiparasitic, through complicated, branched pathways by multiple insecticidal, anti-feedant and allelopathic activities (3)and leaf wax components (4). -

Chandran Et Al. Supporting Info.Pdf
Supporting Information (SI) Appendix Part 1. Impact of tissue preparation, LMD, and RNA amplification on array output. p. 2 Text S1: Detailed Experimental Design and Methods Figure S1A: Correlation analysis indicates tissue preparation has minimal impact on ATH1 array output. Figure S1B: Correlation analysis indicates RNA degradation does not significantly impact array output. Figure S1C: Correlation analysis indicates two-round amplification does not significantly impact array output. Figure S1D: Independent biological replicates of LMD samples are highly correlated. Figure S1E: Validation of LMD array expression by qPCR. Table S1: Tissue preparation-associated genes Part 2. Analysis of LMD and parallel whole leaf array data. p. 13 Table S2A: Known PM-impacted genes enriched in LMD dataset Table S2B: Dataset of LMD and whole leaf genes with PM-altered expression Table S2C: LMD PM MapMan Results and Bins Table S2D: ics1 vs. WT LMD PM MapMan Results and Bins Table S2E: Infection site-specific changes for redox and calcium categories Table S2F: cis-acting regulatory element motif analysis Part 3. Process network construction p. 149 3A. Photosynthesis 3B. Cold/dehydration response Part 4. Powdery mildew infection of WT and myb3r4 mutants p. 173 Text S4: Detailed Experimental Design and Methods (supplement to manuscript) Figure S4A. Uninfected 4 week old WT and myb3r4 plants Figure S4B. myb3r4 mutants exhibit reduced visible PM growth and reproduction Figure S4C. PM-infected WT and myb3r4 mutants do not exhibit cell death Figure S4D. Endoreduplication occurs at site of PM infection not distal to infection Figure S4E. Ploidy correlates with nuclear size. Part 1. Impact of tissue preparation, LMD, and RNA amplification on array output.