AESA Based IPM – Brinjal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Insect Pests of Vegetables
DATE- 18/03/2020 (LECTURE 3) Insect Pests of Vegetables Pests of brinjal Shoot and fruit borer (Leuciniodes orbonalis Guenee, Lepidoptera: Pyralidae) Key Identification- The Major host plants of the pest is Brinjal This pest is reported from regions of brinjal cultivation in Africa, South of the Sahara, and South-East Asia including India, Bangladesh, Srilanka, China and Philippines. Young caterpillars are creamy white, while full-grown are light pinkish and measure about 18-23 mm in length. The moth is medium sized with white wings having triangular brown and red markings on the forewings. The moth measures bout 20-22 mm across the wings. Life cycle- A female moth lays about 80-120 creamy white eggs, singly or in batches of 2-4 on the underside of leaves, on green stems, flowers buds or the calyces of fruits during its life span of 2-5 days. The incubation period is 3-6 days. The young caterpillars bore into the tender shoots near the growing points, flower buds or the fruits. There are five larval instars and mature in 14-20 days, depending on the prevailing temperature and humidity. A single caterpillar may destroy as many as 4-6 fruits. The larva comes out of the damaged shoot or fruit and pupates in a tough gray cocoon in soil or plant debris. The pupal period is 6-11 days. During the active season, the total life cycle is completed in 20-43 days. There are five overlapping generations in a year. Damage- The larvae bore into the petiole and midribs of large leaves and tender shoots and cause wilting. -

Major Insect Pests of Brinjal and Their Management
Popular Article Popular Kheti Volume -4, Issue-2 (April -June), 2016 Available online at www.popularkhet i.com © 2016 popularkheti.com eISSN: 2321-0001 Major Insect Pests of Brinjal a nd Their Management Kanchan G. Padwal 1*, Sameer Kumar Singh 1, Sujeet Kumar Sharma 2 1Department of Entomology and Agricultural Zoology Institute of Agricultural Sciences , Banaras Hi ndu University, Varanasi (UP) 2Division of Plant Protection , Indian Institute of Vegetable Research, Varanasi (UP) *Email of corresponding author : [email protected] Brinjal is the most common and extensively grown all over the country and occupy an important place in the food basket of Indian consumers and attacked by many insect pests. Among them some have been problem for brinjal cultivation. The present article gives emphasis on the identification, life cycle, nature of damage and susta inable management of major insect pests of the brinjal. Introduction Brinjal ( Solanum melongena L.), also known as egg plant is referred as “King of vegetables”, originated from India and now grown as a vegetable throughout the tropical, sub -tropical and warm temperate areas of the world. Eggplant contains nutrients such as dietary fiber, folate, ascorbic acid, vitamin K, niacin, vitamin B6, pantothenic acid, potassium, iron, magnesium, manganese, phosphorus , and copper; the nutrients that it contributes to the diets of the poor are especially important during times when other vegetables are in short supply. The crop is attacked by number of insect pests including Leafhopper (Amrasca devastans ), aphid ( Aphis gossypii ), white fly ( Bemisia tabaci ) and shoot and fruit borer ( Leucinodes orbonalis ), Hadda beetle (Epilachna vigintioctopunctata ), Stem borer ( Euzophera perticella ), Lace wing bug ( Urentius hystricellus ), Brinjal brown leaf hopper ( Cestius phycitis ), Leaf roller ( Eublemma olivacea ).Besides these insect pests brinjal is also attacked by several other pests including mites, which result in significant losses in the yield. -

Insect Morphology and Systematics (Ento-131) - Notes
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/276175248 Insect Morphology and Systematics (Ento-131) - Notes Book · April 2010 CITATIONS READS 0 14,110 1 author: Cherukuri Sreenivasa Rao National Institute of Plant Health Management (NIPHM), Hyderabad, India 36 PUBLICATIONS 22 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Agricultural College, Jagtial View project ICAR-All India Network Project on Pesticide Residues View project All content following this page was uploaded by Cherukuri Sreenivasa Rao on 12 May 2015. The user has requested enhancement of the downloaded file. Insect Morphology and Systematics ENTO-131 (2+1) Revised Syllabus Dr. Cherukuri Sreenivasa Rao Associate Professor & Head, Department of Entomology, Agricultural College, JAGTIAL EntoEnto----131131131131 Insect Morphology & Systematics Prepared by Dr. Cherukuri Sreenivasa Rao M.Sc.(Ag.), Ph.D.(IARI) Associate Professor & Head Department of Entomology Agricultural College Jagtial-505529 Karminagar District 1 Page 2010 Insect Morphology and Systematics ENTO-131 (2+1) Revised Syllabus Dr. Cherukuri Sreenivasa Rao Associate Professor & Head, Department of Entomology, Agricultural College, JAGTIAL ENTO 131 INSECT MORPHOLOGY AND SYSTEMATICS Total Number of Theory Classes : 32 (32 Hours) Total Number of Practical Classes : 16 (40 Hours) Plan of course outline: Course Number : ENTO-131 Course Title : Insect Morphology and Systematics Credit Hours : 3(2+1) (Theory+Practicals) Course In-Charge : Dr. Cherukuri Sreenivasa Rao Associate Professor & Head Department of Entomology Agricultural College, JAGTIAL-505529 Karimanagar District, Andhra Pradesh Academic level of learners at entry : 10+2 Standard (Intermediate Level) Academic Calendar in which course offered : I Year B.Sc.(Ag.), I Semester Course Objectives: Theory: By the end of the course, the students will be able to understand the morphology of the insects, and taxonomic characters of important insects. -

Morphological and Biochemical Characters of Eggplant
Journal of Entomology and Zoology Studies 2018; 6(5): 915-920 E-ISSN: 2320-7078 P-ISSN: 2349-6800 Morphological and biochemical characters of JEZS 2018; 6(5): 915-920 © 2018 JEZS eggplant (Solanum melongena) conferring Received: 09-07-2018 Accepted: 10-08-2018 resistance against whitefly (Bemisia tabaci) Muhammad Musa Khan Department of Entomology, University of Agriculture Muhammad Musa Khan, Muhammad Yahya Khan, Rana Muhammad Faisalabad, Pakistan Kaleem Ullah, Muhammad Yasir, Annum Khalid and Muhammad Ahsan Muhammad Yahya Khan Khan Department of Entomology, University of Agriculture Abstract Faisalabad, Pakistan Results showed that maximum whitefly incidence was observed in Black Round and the minimum population was recorded on Black Beauty. Correlation analysis described that the hair length on midrib Rana Muhammad Kaleem Ullah region, leaf lamina thickness and percentage of manganese contents showed negative and hair density on Department of Entomology, Faculty of Agriculture Science midrib region, nitrogen contents and protein contents have a positive correlation with whitefly and Technology, Bahauddin population. When the morphological characters were regressed together, hair density on midrib was a Zakariya University Multan. most important parameter with 72.1% impact. The combination of all morphological parameters has no Pakistan significant impact on the whitefly population. Among all the biochemical parameters nitrogen showed maximum 71.1% impact while all parameters together showed 8.4% impact. Principle component Muhammad Yasir analysis showed that the thickness of the leaf lamina played an important role in the reduction of whitefly Department of Horticulture, when all significantly correlated parameters were calculated together. From the results, it can be Agriculture University concluded that the morphological and biochemical characters of brinjal imparted resistance against Peshawar, Pakistan whitefly which can be used as an important tool in integrated pest management model. -

Screening of Different Aubergine Cultivars Against Infestation of Brinjal Fruit and Shoot Borer (Leucinodes Orbonalis Guenee)
Pakistan J. Zool., vol. 51(2), pp 603-609, 2019. DOI: http://dx.doi.org/10.17582/journal.pjz/2019.51.2.603.609 Screening of Different Aubergine Cultivars against Infestation of Brinjal Fruit and Shoot Borer (Leucinodes orbonalis Guenee) Ajmal Khan Kassi1*, Humayun Javed1 and Tariq Mukhtar2 1Department of Entomology, Pir Mehr Ali Shah-Arid Agriculture University, Rawalpindi 2Department of Plant Pathology, Pir Mehr Ali Shah-Arid Agriculture University, Rawalpindi ABSTRACT Article Information Received 27 March 2018 In the present study, response of five aubergine cultivars was evaluated against Brinjal Fruit and Shoot Revised 12 May 2018 Borer (Leucinodes orbonalis Guenee) under field conditions. The cultivar Round White Brinjal showed Accepted 27 June 2018 maximum fruit infestation (54.44%) followed by Singhnath 666 (53.19%) while the minimum fruit Available online 15 February 2019 infestation was observed on Round Brinjal 86602 (42.39%). Contrarily, Short Purple cultivar showed Authors’ Contribution maximum larval population (0.43) followed by Round White Brinjal (0.39) while the minimum larval AKK, HJ and TM designed the study population was observed on Round Brinjal 86602 (0.27) and was found comparatively resistant against and executed the experimental work. the pest. The correlation between fruit infestation and larval population and different environmental TM analyzed the data. HJ and TM factors was also studied. Average relative humidity was found positive and significantly correlated supervised the work and helped in with fruit infestation. Average precipitation showed positive but non-significant correlation in case of preparation of the manuscript. all the cultivars except Singhnath 666 which was positive and significant. -

M.Sc. Zoology
CMS COLLEGE KOTTAYAM (AUTONOMOUS) Affiliated to the Mahatma Gandhi University Kottayam, Kerala CURRICULUM FOR POST GRADUATE PROGRAMME MASTER OF SCIENCE IN ZOOLOGY UNDER CREDIT AND SEMESTER SYSTEM (CSS) (With effect from 2019 Admissions) Approved by the Board of Studies on 10th April 2019 CONTENTS 1. Board of Studies 2. Acknowledgements 3. Preface 4. Academic Regulations 5. Curriculum a. Graduate Programme Outcome b. Programme Specific Outcome 6. Programme Design 7. Programme Structure Semester wise 8. Detailed Syllabus of the courses II BOARD OF STUDIES Dr. Sosamma Oommen (Chairman) Head of the Department, Zoology CMS College Kottayam (Autonomous) Dr. Johnson Baby (Subject Expert) Principal Christian College Chengannur Dr. Nagendra Prabhu (Subject Expert) Associate Professor Department of Zoology Sanatana Dharma College Alleppey Dr. A.P Thomas (University Nominee) Director, ACESSD M G University Kottayam Dr. Maya B Nair (Alumni Representative) Assistant professor Department of Zoology Sanatana Dharma College Alleppey Mr. Ajesh James (Beneficiary Representative) CPC certified Professional Coder International Institute of Medical Coding (AAPC approved), 188A, CMS College Road, Kottayam Dr. Jobin Mathew (Member) Assistant Professor Department of Zoology CMS College Kottayam (Autonomous) Dr. Nisha P Aravind (Member) Assistant Professor Department of Zoology CMS College Kottayam (Autonomous) Dr. Pushpa Geetha S (Member) Assistant Professor Department of Zoology CMS College Kottayam (Autonomous) III Prof. Vijo Thomas Kurien (Member Secretary) -

(Leucinodes Orbonalis Guenee) on Eggplant (Solanum Melongena Linnaeus): a Review
International Journal of Entomology Research International Journal of Entomology Research ISSN: 2455-4758 Impact Factor: RJIF 5.24 www.entomologyjournals.com Volume 3; Issue 2; March 2018; Page No. 28-33 Life aspects and mode of damage of brinjal shoot and fruit borer (Leucinodes orbonalis Guenee) on eggplant (Solanum melongena Linnaeus): A review * Muhammad Abdullah Shaukat, Ahmad Manan Mustafa, Ammad Ahmad, Sohail Maqsood, Umer Hayat, Farwa Mustafa, Gulraiz Malik Department of Entomology, University College of Agriculture and Environmental Sciences, The Islamia University of Bahawalpur, Punjab, Pakistan Abstract Brinjal (Eggplant) Solanum melongena Linnaeus is the most important vegetable of hot and wet climatic zones. It is commercially very accessible and profitable vegetable to farmers. A wide range of essential biochemicals and minerals belongs to brinjal including vitamins, proteins, calcium, phosphorus. Brinjal Shoot and Fruit Borer Leucinodes orbonalis Guenee is the major infectious insect causing a high toll to plants. BSFB generally depends on brinjal but sometimes turns towards other solanaceous field crops and may be on wild hosts. The pest is spreader to wide areas of eggplant cultivation with South of Sahara, Africa and Asia including China and Philippines. Egg-laying occurs during night and incubation period ranges from 3-8 days depending of environmental conditions. Larval period completes in 12-22 days depending upon environmental situations and passes through five instars. Full grown larvae pupate into the soil or under plant debris and dropped dead shoots. Adult of BSFB is a whitish moth which hide during day time and activates from dusk to perform various activities like mating oviposition. It was investigated that environmental factors have a great impact on the life of L. -
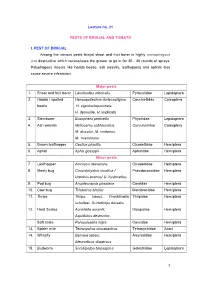
Lecture No. 21
Lecture no. 21 PESTS OF BRINJAL AND TOMATO I. PEST OF BRINJAL Among the various pests brinjal shoot and fruit borer is highly monophagous and destructive which necessitates the grower to go in for 30 - 40 rounds of sprays. Polyphagous insects like hadda beetle, ash weevils, leafhoppers and aphids also cause severe infestation. Major pests 1. Shoot and fruit borer Leucinodes orbonalis Pyraustidae Lepidoptera 2. Hadda / spotted Henosepilachna dodecastigma, Coccinellidae Coleoptera beetle H. vigintioctopunctata, H. demurille, H. implicata 3. Stemborer Euzophera perticella Phycitidae Lepidoptera 4. Ash weevils Myllocerus subfasciatus, Curculionidae Coleoptera M. discolor, M. viridanus, M. maculosus 5. Brown leafhopper Cestius phycitis Cicadellidae Hemiptera 6. Aphid Aphis gossypii Aphididae Hemiptera Minor pests 7. Leafhopper Amrasca devastans Cicadellidae Hemiptera 8. Mealy bug Coccidohystrix insolitus / Pseudococcidae Hemiptera Urentius ectinus/ U. hystricellus 9. Pod bug Anoplecnemis phasiana Coreidae Hemiptera 10. Cow bug Tricentrus bicolor Membracidae Hemiptera 11. Thrips Thrips tabaci, Frankliniella Thripidae Hemiptera schultzei, Scirtothrips dorsalis 12. Hard Scales Aonidiella aurantii, Diaspidiae Hemiptera Aspidiotus destructor, Soft scale Parasaissetia nigra Coccidae Hemiptera 13. Spider mite Tetranychus cinnabarinus Tetranychidae Acari 14. Whitefly Bemisia tabaci, Aleyrodidae Hemiptera Aleurodicus dispersus 15. Budworm Scrobipalpa blapsigona Gelechiidae Lepidoptera 16. Leaf roller Antoba olivacea Noctuidae Lepidoptera 17. Leaf -

Diversity of Insect Pests and Their Natural Enemies in Brinjal
Int.J.Curr.Microbiol.App.Sci (2018) 7(7): 3714-3717 International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 7 Number 07 (2018) Journal homepage: http://www.ijcmas.com Original Research Article https://doi.org/10.20546/ijcmas.2018.707.429 Diversity of Insect Pests and their Natural Enemies in Brinjal R. Kumar, M.K. Mahla, G. Chhangani*, B. Singh and V. Kumar Department of Entomology, Rajasthan College of Agriculture (MPUAT) Udaipur, India *Corresponding author ABSTRACT K e yw or ds The investigation on, “diversity of insect pests and natural enemies in brinjal” was conducted at Horticulture Farm and Department of Entomology, Rajasthan College of Brinjal, Biodiversity, Insect Agriculture, Udaipur during kharif 2015. The seedling of brinjal variety “Kavach” was pests, Natural transplanted to record the different insect pests population and natural enemies diversity in enemies, Relative the brinjal ecosystem. The diversity of insect pests infesting brinjal during 2015-16 density comprised four species of sap sucking insects, the aphids, jassids, whiteflies and tinged bugs; the shoot and fruit borer and hadda beetle .On the basis of mean density, whiteflies Article Info had the maximum seasonal mean density 20.11 per 5 plants followed by aphids and jassids. The relative density was also the maximum (25.78) for whiteflies on account of Accepted: their population abundance. Among the common natural enemies associated with their 26 June 2018 Available Online: pests the maximum mean density (15.69) and relative density (42.91) was for the lady bird 10 July 2018 beetle; however, syrphid fly and spiders were also recorded. -

Insect and Mite Pests Diversity in the Important Vegetable Crops Ecosystems in Bangladesh
Insect and Mite Pests Diversity in the Important Vegetable Crops Ecosystems in Bangladesh G.P. Das, Ph.D. IUCN-The World Conservation Union Bangladesh Country Office June 2004 UICN Bibliothaque CH - 11 96 Gland The materials in this publication may be reproduced in whole or in part and in any form for educational or non-profit purposes, without special permission from the copyright holder, provided proper acknowledgement of the source is made. IUCN Bangladesh would appreciate receiving a copy of any publication, which uses this document as a source. Foreword The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN concerning the legal status of any country, territory, administration, or concerning the delimitation of its frontiers or boundaries. Biodiversity or, the diversity of life has qlways fascinated people everywhere. It constitutes the outcome of the evolutionary processes of living organisms. This publication may not be resold or used for any other commercial purpose without the prior written permission of IUCN Bangladesh. The numbers of the flora and the fauna and their diversities increased geometrically through perhaps 2.5 billion years, proliferating by biological Published by: IUCN Bangladesh Country Office processes, and controlled by natural selection, filling almost every habitable IUCN ecological niche all around us. Biodiversity, also called "natural capital" consists flt WN1• Ctwrl1l111 l1ln of every form of life from microbes to the mightiest beasts and the gigantic Copyright: © 2004, IUCN-The World Conservation Union trees. Bangladesh with her warm and humid climate is very rich in biodiversity Reproduction of this publication for educational or other non including mammals, birds, reptiles, amphibians, marine and freshwater commercial purposes is authorized without prior written species, angiosperms, soil organisms, insects, etc. -

Study Material of Pests of Horticultural Crops and Their Management Ag
STUDY MATERIAL OF PESTS OF HORTICULTURAL CROPS AND THEIR MANAGEMENT AG. ENTO. 4.4 (2 +1) FOR POLYTECHNIC IN AGRICULTURE STUDENTS COMPILED BY Dr. D. R. Patel, Dr. J. J. Patel, Mr. D. V. Muchhadiya and Mr.R.B.patel POLYTECHNIC IN AGRICULTURE NAVSARI AGRICULTURAL UNIVERSITY BHARUCH– 392 012 Ag. Ento. 4.4 Pests of Horticultural Crops and their Management Credit hours: 3 (2+1) Theory Distribution, biology, nature and symptoms of damage, and management strategies of major insect and non-insect pests of vegetable crops (brinjal, okra, tomato, potato, chilies, onion and garlic) cruciferous and cucurbitaceous vegetables crops (cabbage, cauliflower, radish and guards crops), fruit trees (mango, sapota, citrus, banana, pomegranate, custard apple, aonla, ber, guava, papaya, coconut and date palm), Flowering plants (rose, marigold and gallardia). Plant protection in protected cultivation. Practicals 1. Identification and nature of damage of pests of solanaceous crops 2. Identification and nature of damage of pests of malvaceous crops 3. Identification and nature of damage of pests of cruciferous crops 4. Identification and nature of damage of pests of cucurbitaceous crops 5. Identification and nature of damage of pests of garlic, turmeric and ginger 6. Identification and nature of damage of pests of pulse vegetable 7. Identification and nature of damage of pests of leafy vegetables 8. Identification and nature of damage of pests of plantation crops (mango and sapota, banana, guava, pomegranate and custard apple; citrus, ber, papaya, moringa and aonla; coconut and date palm) 9. Identification and nature of damage of pests of rose, marigold and gallardia Ag. Ento. 4.4 Pests of Horticultural Crops and their Management Credits 3(2+1) Vegetables are increasingly becoming important for nutritional and livelihood security due to nutritional richness, economic viability and ability to generate on-farm and off-farm employment. -

Abundance of Insect Pests and Their Natural Enemies Associated with Brinjal (Solanum Melongena) Crop
Reviews In Food And Agriculture (RFNA) 2(1) (2021) 01-03 Reviews In Food And Agriculture (RFNA) DOI: http://doi.org/10.26480/rfna.01.2021.01.03 ISSN: 2735-0312 (Online) CODEN: RFAEAW RESEARCH ARTICLE ABUNDANCE OF INSECT PESTS AND THEIR NATURAL ENEMIES ASSOCIATED WITH BRINJAL (SOLANUM MELONGENA) CROP Muhammad Ramzana*, Ghulam Murtazab, Muhammad Naumanc, Aqsa Zainabd, Ahmad Alie, Muhammad Umaire, Mamoon Shafiqe aState Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing 100193, China bDepartment of Entomology, College of Plant Protection, China Agricultural University, Beijing, China cInstitute of Plant Protection, MNS University of Agriculture, Multan dDepartment of English Language and Literature, University of Punjab Lahore Pakistan eDepartment of Entomology, University of Agriculture, Faisalabad *Corresponding author email: [email protected] This is an open access article distributed under the Creative Commons Attribution License CC BY 4.0, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ARTICLE DETAILS ABSTRACT Article History: An experimental study was conducted to check the abundance of insect pests and biological fauna in brinjal crop (Solanum melongena L.) during 2018. One acre of brinjal was cultivated for this purpose and data was Received 10 October 2020 recorded on weekly basis from ten tagged plant. In this study, biological fauna such as hover fly, honey bee, Accepted 12 November 2020 Available online 2020 butterfly, green lacewing, praying mantis and ladybird beetle were recorded. The insect pests such as brinjal fruit borer, leafhopper, whitefly, leaf roller, thrips, stem borer, aphid and mealybug were recorded during the 30 November present study.