Download Download
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Insect Orders V: Panorpida & Hymenoptera
Insect Orders V: Panorpida & Hymenoptera • The Panorpida contain 5 orders: the Mecoptera, Siphonaptera, Diptera, Trichoptera and Lepidoptera. • Available evidence clearly indicates that the Lepidoptera and the Trichoptera are sister groups. • The Siphonaptera and Mecoptera are also closely related but it is not clear whether the Siponaptera is the sister group of all of the Mecoptera or a group (Boreidae) within the Mecoptera. If the latter is true, then the Mecoptera is paraphyletic as currently defined. • The Diptera is the sister group of the Siphonaptera + Mecoptera and together make up the Mecopteroids. • The Hymenoptera does not appear to be closely related to any of the other holometabolous orders. Mecoptera (Scorpionflies, hangingflies) • Classification. 600 species worldwide, arranged into 9 families (5 in the US). A very old group, many fossils from the Permian (260 mya) onward. • Structure. Most distinctive feature is the elongated clypeus and labrum that together form a rostrum. The order gets its common name from the gential segment of the male in the family Panorpodiae, which is bulbous and often curved forward above the abdomen, like the sting of a scorpion. Larvae are caterpillar-like or grub- like. • Natural history. Scorpionflies are most common in cool, moist habitats. They get the name “hangingflies” from their habit of hanging upside down on vegetation. Larvae and adult males are mostly predators or scavengers. Adult females are usually scavengers. Larvae and adults in some groups may feed on vegetation. Larvae of most species are terrestrial and caterpillar-like in body form. Larvae of some species are aquatic. In the family Bittacidae males attract females for mating by releasing a sex pheromone and then presenting the female with a nuptial gift. -

BÖCEKLERİN SINIFLANDIRILMASI (Takım Düzeyinde)
BÖCEKLERİN SINIFLANDIRILMASI (TAKIM DÜZEYİNDE) GÖKHAN AYDIN 2016 Editör : Gökhan AYDIN Dizgi : Ziya ÖNCÜ ISBN : 978-605-87432-3-6 Böceklerin Sınıflandırılması isimli eğitim amaçlı hazırlanan bilgisayar programı için lütfen aşağıda verilen linki tıklayarak programı ücretsiz olarak bilgisayarınıza yükleyin. http://atabeymyo.sdu.edu.tr/assets/uploads/sites/76/files/siniflama-05102016.exe Eğitim Amaçlı Bilgisayar Programı ISBN: 978-605-87432-2-9 İçindekiler İçindekiler i Önsöz vi 1. Protura - Coneheads 1 1.1 Özellikleri 1 1.2 Ekonomik Önemi 2 1.3 Bunları Biliyor musunuz? 2 2. Collembola - Springtails 3 2.1 Özellikleri 3 2.2 Ekonomik Önemi 4 2.3 Bunları Biliyor musunuz? 4 3. Thysanura - Silverfish 6 3.1 Özellikleri 6 3.2 Ekonomik Önemi 7 3.3 Bunları Biliyor musunuz? 7 4. Microcoryphia - Bristletails 8 4.1 Özellikleri 8 4.2 Ekonomik Önemi 9 5. Diplura 10 5.1 Özellikleri 10 5.2 Ekonomik Önemi 10 5.3 Bunları Biliyor musunuz? 11 6. Plocoptera – Stoneflies 12 6.1 Özellikleri 12 6.2 Ekonomik Önemi 12 6.3 Bunları Biliyor musunuz? 13 7. Embioptera - webspinners 14 7.1 Özellikleri 15 7.2 Ekonomik Önemi 15 7.3 Bunları Biliyor musunuz? 15 8. Orthoptera–Grasshoppers, Crickets 16 8.1 Özellikleri 16 8.2 Ekonomik Önemi 16 8.3 Bunları Biliyor musunuz? 17 i 9. Phasmida - Walkingsticks 20 9.1 Özellikleri 20 9.2 Ekonomik Önemi 21 9.3 Bunları Biliyor musunuz? 21 10. Dermaptera - Earwigs 23 10.1 Özellikleri 23 10.2 Ekonomik Önemi 24 10.3 Bunları Biliyor musunuz? 24 11. Zoraptera 25 11.1 Özellikleri 25 11.2 Ekonomik Önemi 25 11.3 Bunları Biliyor musunuz? 26 12. -
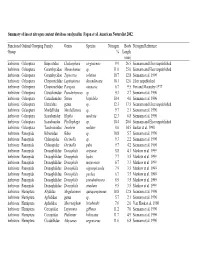
Summary of Insect Nitrogen Content Database Analyzed in Fagan Et Al
Summary of insect nitrogen content database analyzed in Fagan et al. American Naturalist 2002. Functional Ordinal Grouping Family Genus Species Nitrogen Body Nitrogen Reference Group % Length (mm) herbivore Coleoptera Buprestidae Chalcophora virginiensis 9.9 26.5 Siemann and Elser unpublished herbivore Coleoptera Cerambycidae Monochamus sp. 11.0 25.0 Siemann and Elser unpublished herbivore Coleoptera Cerambycidae Typocerus velutina 10.7 12.8 Siemann et al. 1996 herbivore Coleoptera Chrysomelidae Leptinotarsa decemlineata 10.1 12.0 Elser unpublished herbivore Coleoptera Chrysomelidae Paropsis atomaria 6.7 9.5 Fox and Macauley 1977 herbivore Coleoptera Curculionidae Pseudorhyncus sp. 9.3 2.7 Siemann et al. 1996 herbivore Coleoptera Curculionidae Sitona hispidula 10.4 4.0 Siemann et al. 1996 herbivore Coleoptera Elateridae genus sp. 12.3 17.5 Siemann and Elser unpublished herbivore Coleoptera Mordellidae Mordellistena sp. 9.7 2.1 Siemann et al. 1996 herbivore Coleoptera Scarabaeidae Hoplia modesta 12.3 6.8 Siemann et al. 1996 herbivore Coleoptera Scarabaeidae Phyllophaga sp. 10.4 20.0 Siemann and Elser unpublished herbivore Coleoptera Tenebrionidae Tenebrio molitor 8.0 14.5 Barker et al. 1998 herbivore Panorpida Bibionidae Bibio sp. 10.8 5.7 Siemann et al. 1996 herbivore Panorpida Chloropidae Oscinella sp. 9.3 2.2 Siemann et al. 1996 herbivore Panorpida Chloropidae Oscinella pube 9.7 4.2 Siemann et al. 1996 herbivore Panorpida Drosophilidae Drosophila arizonae 8.8 4.1 Markow et al. 1999 herbivore Panorpida Drosophilidae Drosophila hydei 7.7 3.5 Markow et al. 1999 herbivore Panorpida Drosophilidae Drosophila mojavensis 6.7 3.3 Markow et al. 1999 herbivore Panorpida Drosophilidae Drosophila nigrospiracula 7.9 3.5 Markow et al. -

Arthropods of Elm Fork Preserve
Arthropods of Elm Fork Preserve Arthropods are characterized by having jointed limbs and exoskeletons. They include a diverse assortment of creatures: Insects, spiders, crustaceans (crayfish, crabs, pill bugs), centipedes and millipedes among others. Column Headings Scientific Name: The phenomenal diversity of arthropods, creates numerous difficulties in the determination of species. Positive identification is often achieved only by specialists using obscure monographs to ‘key out’ a species by examining microscopic differences in anatomy. For our purposes in this survey of the fauna, classification at a lower level of resolution still yields valuable information. For instance, knowing that ant lions belong to the Family, Myrmeleontidae, allows us to quickly look them up on the Internet and be confident we are not being fooled by a common name that may also apply to some other, unrelated something. With the Family name firmly in hand, we may explore the natural history of ant lions without needing to know exactly which species we are viewing. In some instances identification is only readily available at an even higher ranking such as Class. Millipedes are in the Class Diplopoda. There are many Orders (O) of millipedes and they are not easily differentiated so this entry is best left at the rank of Class. A great deal of taxonomic reorganization has been occurring lately with advances in DNA analysis pointing out underlying connections and differences that were previously unrealized. For this reason, all other rankings aside from Family, Genus and Species have been omitted from the interior of the tables since many of these ranks are in a state of flux. -

Insects of Larose Forest (Excluding Lepidoptera and Odonates)
Insects of Larose Forest (Excluding Lepidoptera and Odonates) • Non-native species indicated by an asterisk* • Species in red are new for the region EPHEMEROPTERA Mayflies Baetidae Small Minnow Mayflies Baetidae sp. Small minnow mayfly Caenidae Small Squaregills Caenidae sp. Small squaregill Ephemerellidae Spiny Crawlers Ephemerellidae sp. Spiny crawler Heptageniiidae Flatheaded Mayflies Heptageniidae sp. Flatheaded mayfly Leptophlebiidae Pronggills Leptophlebiidae sp. Pronggill PLECOPTERA Stoneflies Perlodidae Perlodid Stoneflies Perlodid sp. Perlodid stonefly ORTHOPTERA Grasshoppers, Crickets and Katydids Gryllidae Crickets Gryllus pennsylvanicus Field cricket Oecanthus sp. Tree cricket Tettigoniidae Katydids Amblycorypha oblongifolia Angular-winged katydid Conocephalus nigropleurum Black-sided meadow katydid Microcentrum sp. Leaf katydid Scudderia sp. Bush katydid HEMIPTERA True Bugs Acanthosomatidae Parent Bugs Elasmostethus cruciatus Red-crossed stink bug Elasmucha lateralis Parent bug Alydidae Broad-headed Bugs Alydus sp. Broad-headed bug Protenor sp. Broad-headed bug Aphididae Aphids Aphis nerii Oleander aphid* Paraprociphilus tesselatus Woolly alder aphid Cicadidae Cicadas Tibicen sp. Cicada Cicadellidae Leafhoppers Cicadellidae sp. Leafhopper Coelidia olitoria Leafhopper Cuernia striata Leahopper Draeculacephala zeae Leafhopper Graphocephala coccinea Leafhopper Idiodonus kelmcottii Leafhopper Neokolla hieroglyphica Leafhopper 1 Penthimia americana Leafhopper Tylozygus bifidus Leafhopper Cercopidae Spittlebugs Aphrophora cribrata -

About the Book the Format Acknowledgments
About the Book For more than ten years I have been working on a book on bryophyte ecology and was joined by Heinjo During, who has been very helpful in critiquing multiple versions of the chapters. But as the book progressed, the field of bryophyte ecology progressed faster. No chapter ever seemed to stay finished, hence the decision to publish online. Furthermore, rather than being a textbook, it is evolving into an encyclopedia that would be at least three volumes. Having reached the age when I could retire whenever I wanted to, I no longer needed be so concerned with the publish or perish paradigm. In keeping with the sharing nature of bryologists, and the need to educate the non-bryologists about the nature and role of bryophytes in the ecosystem, it seemed my personal goals could best be accomplished by publishing online. This has several advantages for me. I can choose the format I want, I can include lots of color images, and I can post chapters or parts of chapters as I complete them and update later if I find it important. Throughout the book I have posed questions. I have even attempt to offer hypotheses for many of these. It is my hope that these questions and hypotheses will inspire students of all ages to attempt to answer these. Some are simple and could even be done by elementary school children. Others are suitable for undergraduate projects. And some will take lifelong work or a large team of researchers around the world. Have fun with them! The Format The decision to publish Bryophyte Ecology as an ebook occurred after I had a publisher, and I am sure I have not thought of all the complexities of publishing as I complete things, rather than in the order of the planned organization. -

Hypothesis on Monochromatic Vision in Scorpionflies Questioned by New
www.nature.com/scientificreports OPEN Hypothesis on monochromatic vision in scorpionfies questioned by new transcriptomic data Received: 7 July 2017 Alexander Böhm 1, Karen Meusemann2,3,4, Bernhard Misof3 & Günther Pass1 Accepted: 12 June 2018 In the scorpionfy Panorpa, a recent study suggested monochromatic vision due to evidence of only a Published: xx xx xxxx single opsin found in transcriptome data. To reconsider this hypothesis, the present study investigates opsin expression using transcriptome data of 21 species including representatives of all major lineages of scorpionfies (Mecoptera) and of three families of their closest relatives, the feas (Siphonaptera). In most mecopteran species investigated, transcripts encode two opsins with predicted peak absorbances in the green, two in the blue, and one in the ultraviolet spectral region. Only in groups with reduced or absent ocelli, like Caurinus and Apteropanorpa, less than four visual opsin messenger RNAs have been identifed. In addition, we found a Rh7-like opsin in transcriptome data derived from larvae of the mecopteran Nannochorista, and in two fea species. Peropsin expression was observed in two mecopterans. In light of these new data, we question the hypothesis on monochromatic vision in the genus Panorpa. In a broader phylogenetic perspective, it is suggested that the common ancestor of the monophyletic taxon Antliophora (Diptera, Mecoptera and Siphonaptera) possessed the full set of visual opsins, a Rh7-like opsin, and in addition a pteropsin as well as a peropsin. In the course of evolution individual opsins were likely lost in several lineages of this clade. Colour vision has two prerequisites1,2: receptors with diferent spectral responses and a neural system that can process their output in a way that preserves colour information. -

Mecoptera: Meropeidae): Simply Dull Or Just Inscrutable?
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Center for Systematic Entomology, Gainesville, Insecta Mundi Florida 8-24-2007 Etymology of the earwigfly, Merope tuber Newman (Mecoptera: Meropeidae): Simply dull or just inscrutable? Louis A. Somma University of Florida, [email protected] James C. Dunford University of Florida, Gainesville, FL Follow this and additional works at: https://digitalcommons.unl.edu/insectamundi Part of the Entomology Commons Somma, Louis A. and Dunford, James C., "Etymology of the earwigfly, Merope tuber Newman (Mecoptera: Meropeidae): Simply dull or just inscrutable?" (2007). Insecta Mundi. 65. https://digitalcommons.unl.edu/insectamundi/65 This Article is brought to you for free and open access by the Center for Systematic Entomology, Gainesville, Florida at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Insecta Mundi by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. INSECTA MUNDI A Journal of World Insect Systematics 0013 Etymology of the earwigfly, Merope tuber Newman (Mecoptera: Meropeidae): Simply dull or just inscrutable? Louis A. Somma Department of Zoology PO Box 118525 University of Florida Gainesville, FL 32611-8525 [email protected] James C. Dunford Department of Entomology and Nematology PO Box 110620, IFAS University of Florida Gainesville, FL 32611-0620 [email protected] Date of Issue: August 24, 2007 CENTER FOR SYSTEMATIC ENTOMOLOGY, INC., Gainesville, FL Louis A. Somma and James C. Dunford Etymology of the earwigfly, Merope tuber Newman (Mecoptera: Meropeidae): Simply dull or just inscrutable? Insecta Mundi 0013: 1-5 Published in 2007 by Center for Systematic Entomology, Inc. P. O. Box 147100 Gainesville, FL 32604-7100 U. -

Annual Report 2008-2009 Annual Report 0
Department of Environment and Conservation and Environment of Department Department of Environment and Conservation 2008-2009 Annual Report 2008-2009 Annual Report Annual 2008-2009 0 ' "p 2009195 E R N M O V E G N T E O H T F W A E I S L T A E R R N A U S T Acknowledgments This report was prepared by the Corporate Communications Branch, Department of Environment and Conservation. For more information contact: Department of Environment and Conservation Level 4 The Atrium 168 St Georges Terrace Perth WA 6000 Locked Bag 104 Bentley Delivery Centre Western Australia 6983 Telephone (08) 6364 6500 Facsimile (08) 6364 6520 Recommended reference The recommended reference for this publication is: Department of Environment and Conservation 2008–2009 Annual Report, Department of Environment and Conservation, 2009. We welcome your feedback A publication feedback form can be found at the back of this publication, or online at www.dec.wa.gov.au. ISSN 1835-1131 (Print) ISSN 1835-114X (Online) 8 September 2009 Letter to THE MINISter Back Contents Forward Hon Donna Faragher MLC Minister for Environment In accordance with section 63 of the Financial Management Act 2006, I have pleasure in submitting for presentation to Parliament the Annual Report of the Department of Environment and Conservation for the period 1 July 2008 to 30 June 2009. This report has been prepared in accordance with provisions of the Financial Management Act 2006. Keiran McNamara Director General DEPARTMENT OF ENVIRONMENT AND CONSERVATION 2008–2009 ANNUAL REPORT 3 DIRECTOR GENERAL’S FOREWORD Back Contents Forward This is the third annual report of the Department of Environment and Conservation since it was created through the merger of the former Department of Environment and Department of Conservation and Land Management. -

Ancient Rapid Radiations of Insects: Challenges for Phylogenetic Analysis
ANRV330-EN53-23 ARI 2 November 2007 18:40 Ancient Rapid Radiations of Insects: Challenges for Phylogenetic Analysis James B. Whitfield1 and Karl M. Kjer2 1Department of Entomology, University of Illinois, Urbana, Illinois 61821; email: jwhitfi[email protected] 2Department of Ecology, Evolution and Natural Resources, Rutgers University, New Brunswick, New Jersey 08901; email: [email protected] Annu. Rev. Entomol. 2008. 53:449–72 Key Words First published online as a Review in Advance on diversification, molecular evolution, Palaeoptera, Orthopteroidea, September 17, 2007 fossils The Annual Review of Entomology is online at ento.annualreviews.org Abstract by UNIVERSITY OF ILLINOIS on 12/18/07. For personal use only. This article’s doi: Phylogenies of major groups of insects based on both morphological 10.1146/annurev.ento.53.103106.093304 and molecular data have sometimes been contentious, often lacking Copyright c 2008 by Annual Reviews. the data to distinguish between alternative views of relationships. Annu. Rev. Entomol. 2008.53:449-472. Downloaded from arjournals.annualreviews.org All rights reserved This paucity of data is often due to real biological and historical 0066-4170/08/0107-0449$20.00 causes, such as shortness of time spans between divergences for evo- lution to occur and long time spans after divergences for subsequent evolutionary changes to obscure the earlier ones. Another reason for difficulty in resolving some of the relationships using molecu- lar data is the limited spectrum of genes so far developed for phy- logeny estimation. For this latter issue, there is cause for current optimism owing to rapid increases in our knowledge of comparative genomics. -

Personal, Qualifications and Employment History
Jonathan Majer - Publications 16 Sept 2018 References marked so, can be obtained by citing reference number in an email to Jonathan Majer ([email protected]) or from DVD provided. Some others can be viewed or downloaded from Curtin eSpace on: http://espace.lis.curtin.edu.au/ Once eSpace is accessed, perform an Advanced search using ‘Majer’ as author. PDF files can be downloaded as required. References highlighted in green are not yet available as PDF files. 1. Books (refereed) Majer, J. D. (ed.) (1989). Animals in Primary Succession. The Role of Fauna in Land Reclamation. Cambridge University Press, Cambridge. 547 pp. #B1 (PDF files available from Jonathan Majer) Agosti, D., Majer, J., Alonso, E. and Schultz, T., (eds.). (2000). Ants: Standard Methods for Measuring and Monitoring Biodiversity. Biological Diversity Handbook Series. Smithsonian Institution Press. Washington D.C., 20+280pp. #B2 (PDF file available from Jonathan Majer) Delabie, J., Fernandez, F & Majer J.(eds.) (2012). Advances in Neotropical Myrmecology. Psyche 2012. #B3 (PDF file available at http://www.hindawi.com/journals/psyche/si/840560/) Gunawardene, N., Majer, J. D., Taylor, C. K., & Harvey, M. S. (eds.) (2013). The Terrestrial Invertebrate Fauna of Barrow Island, Western Australia. Records of the Western Australian Museum. Supplement 83. 406 pp. #B4 (PDF file available from Jonathan Majer) Delabie, J.H.C., Feitosa, R., Serrão, J.E., Mariano, C.S.F. & Majer, J.D. (eds) (2015). Formigas Poneromorfas do Brasil. Editus, Ilhéus (Bahia), Brazil. 450 pp. #B5(PDF file available from Jonathan Majer)Link to chapters: http://books.scielo.org/id/m3gqd Agosti, D., Majer, J., Alonso, E. -

Phylogeny of Endopterygote Insects, the Most Successful Lineage of Living Organisms*
REVIEW Eur. J. Entomol. 96: 237-253, 1999 ISSN 1210-5759 Phylogeny of endopterygote insects, the most successful lineage of living organisms* N iels P. KRISTENSEN Zoological Museum, University of Copenhagen, Universitetsparken 15, DK-2100 Copenhagen 0, Denmark; e-mail: [email protected] Key words. Insecta, Endopterygota, Holometabola, phylogeny, diversification modes, Megaloptera, Raphidioptera, Neuroptera, Coleóptera, Strepsiptera, Díptera, Mecoptera, Siphonaptera, Trichoptera, Lepidoptera, Hymenoptera Abstract. The monophyly of the Endopterygota is supported primarily by the specialized larva without external wing buds and with degradable eyes, as well as by the quiescence of the last immature (pupal) stage; a specialized morphology of the latter is not an en dopterygote groundplan trait. There is weak support for the basal endopterygote splitting event being between a Neuropterida + Co leóptera clade and a Mecopterida + Hymenoptera clade; a fully sclerotized sitophore plate in the adult is a newly recognized possible groundplan autapomorphy of the latter. The molecular evidence for a Strepsiptera + Díptera clade is differently interpreted by advo cates of parsimony and maximum likelihood analyses of sequence data, and the morphological evidence for the monophyly of this clade is ambiguous. The basal diversification patterns within the principal endopterygote clades (“orders”) are succinctly reviewed. The truly species-rich clades are almost consistently quite subordinate. The identification of “key innovations” promoting evolution