Hu Et Al., Afr J Tradit Complement Altern Med. (2016) 13(3):60-65
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2. Ethnic Minority Policy
Public Disclosure Authorized ETHNIC MINORITY DEVELOPMENT PLAN FOR THE WORLD BANK FUNDED Public Disclosure Authorized GANSU INTEGRATED RURAL ECONOMIC DEVELOPMENT DEMONSTRATION TOWN PROJECT Public Disclosure Authorized GANSU PROVINCIAL DEVELOPMENT AND REFORM COMMISSION Public Disclosure Authorized LANZHOU , G ANSU i NOV . 2011 ii CONTENTS 1. INTRODUCTION ................................................................ ................................ 1.1 B ACKGROUND AND OBJECTIVES OF PREPARATION .......................................................................1 1.2 K EY POINTS OF THIS EMDP ..........................................................................................................2 1.3 P REPARATION METHOD AND PROCESS ..........................................................................................3 2. ETHNIC MINORITY POLICY................................................................ .......................... 2.1 A PPLICABLE LAWS AND REGULATIONS ...........................................................................................5 2.1.1 State level .............................................................................................................................5 2.1.2 Gansu Province ...................................................................................................................5 2.1.3 Zhangye Municipality ..........................................................................................................6 2.1.4 Baiyin City .............................................................................................................................6 -

Project Completion Report Apr-16 Sep-16 Oct-18 Dec-18 Plan Actual Cancel
Completion Report Project Number: 43025-013 Loan Number: 2760 October 2020 People’s Republic of China: Gansu Tianshui Urban Infrastructure Development Project This document is being disclosed to the public in accordance with ADB’s Access to Information Policy. CURRENCY EQUIVALENTS Currency unit – yuan (CNY) At Appraisal At Project Completion (2 June 2011) (12 August 2019) CNY1.00 = $0.1544 $0.1416 $1.00 = CNY6.4780 CNY7.0624 ABBREVIATIONS ADB – Asian Development Bank CHP – combined heat and power plant DMF – design and monitoring framework EIA – environmental impact assessment EIRR – economic internal rate of return EMP – environmental management plan FIRR – financial internal rate of return GAP – gender action plan GPG – Gansu Provincial Government GTEZ – Guanzhong–Tianshui Economic Zone LAR – land acquisition and resettlement NMT – nonmotorized transport O&M – operation and maintenance PIA – project implementing agency PRC – People’s Republic of China THC – Tianshui Heating Company TMG – Tianshui Municipal Government TPMO – Tianshui project management office TUCIC – Tianshui Urban Construction and Investment Group Co. Ltd WACC – weighted average cost of capital WEIGHTS AND MEASURES km – kilometer m – meter m2 – square meter m3 – cubic meter mu – 666.67 square meters NOTE In this report, “$” refers to United States dollars. Vice-President Ahmed M. Saeed, Operations 2 Director General James P. Lynch, East Asia Department (EARD) Director Yolanda Fernandez Lommen, People’s Republic of China Resident Mission (PRCM), EARD Team leader Xinjian -

Annual Report 2019
HAITONG SECURITIES CO., LTD. 海通證券股份有限公司 Annual Report 2019 2019 年度報告 2019 年度報告 Annual Report CONTENTS Section I DEFINITIONS AND MATERIAL RISK WARNINGS 4 Section II COMPANY PROFILE AND KEY FINANCIAL INDICATORS 8 Section III SUMMARY OF THE COMPANY’S BUSINESS 25 Section IV REPORT OF THE BOARD OF DIRECTORS 33 Section V SIGNIFICANT EVENTS 85 Section VI CHANGES IN ORDINARY SHARES AND PARTICULARS ABOUT SHAREHOLDERS 123 Section VII PREFERENCE SHARES 134 Section VIII DIRECTORS, SUPERVISORS, SENIOR MANAGEMENT AND EMPLOYEES 135 Section IX CORPORATE GOVERNANCE 191 Section X CORPORATE BONDS 233 Section XI FINANCIAL REPORT 242 Section XII DOCUMENTS AVAILABLE FOR INSPECTION 243 Section XIII INFORMATION DISCLOSURES OF SECURITIES COMPANY 244 IMPORTANT NOTICE The Board, the Supervisory Committee, Directors, Supervisors and senior management of the Company warrant the truthfulness, accuracy and completeness of contents of this annual report (the “Report”) and that there is no false representation, misleading statement contained herein or material omission from this Report, for which they will assume joint and several liabilities. This Report was considered and approved at the seventh meeting of the seventh session of the Board. All the Directors of the Company attended the Board meeting. None of the Directors or Supervisors has made any objection to this Report. Deloitte Touche Tohmatsu (Deloitte Touche Tohmatsu and Deloitte Touche Tohmatsu Certified Public Accountants LLP (Special General Partnership)) have audited the annual financial reports of the Company prepared in accordance with PRC GAAP and IFRS respectively, and issued a standard and unqualified audit report of the Company. All financial data in this Report are denominated in RMB unless otherwise indicated. -

43025-013: Gansu Tianshui Urban Infrastructure Development Project
Environmental Monitoring Report Project Number: 43025-013 July 2018 PRC: Gansu Tianshui Urban Infrastructure Development Project Prepared by Tianshui Project Management Office for Tianshui Municipal Government, Tianshui Environment Protection Bureau, and the Asian Development Bank. This environmental monitoring report is a document of the borrower. The views expressed herein do not necessarily represent those of ADB's Board of Directors, Management, or staff, and may be preliminary in nature. In preparing any country program or strategy, financing any project, or by making any designation of or reference to a particular territory or geographic area in this document, the Asian Development Bank does not intend to make any judgments as to the legal or other status of any territory or area. # 10 Semi-annual Report July 2018 (through January to June 2018) People’s Republic of China: Gansu Tianshui Urban Infrastructure Development Project Prepared by Tianshui Project Management Office(Tianshui Urban Construction and Investment Co.) for the Tianshui Municipal Government, Tianshui Environment Protection Bureau, and the Asian Development Bank. This environmental monitoring report is a document of the borrower. The views expressed herein do not necessarily represent those of ADB's Board of Directors, Management, or staff, and may be preliminary in nature. In preparing any country program or strategy, financing any project, or by making any designation of or reference to a particular territory or geographic area in this document, the Asian Development -

World Bank Document
World Bank-financed Project Public Disclosure Authorized Gansu Revitalization and Innovation Project Social Assessment Report Public Disclosure Authorized Public Disclosure Authorized Gansu Project Management Office Public Disclosure Authorized April 2019 Contents 1. Introduction ........................................................................................................................... 1 1.1 BACKGROUND....................................................................................................................................... 2 1.2 SA TASKS ............................................................................................................................................. 3 1.3 SA METHODS ........................................................................................................................................ 3 1.3.1 Organizational interview and literature collection ............................................................................. 3 1.3.2 FGD ................................................................................................................................................... 4 1.3.3 Key informant interview ..................................................................................................................... 5 1.3.4 Questionnaire survey ........................................................................................................................ 6 1.3.5 Field investigation ............................................................................................................................ -

Buried Gas Pipe Network Project in Residential Communities PE 100+
Buried gas network Case: Buried Gas Pipe Network Project in Residential Communities PE 100+ member: PetroChina Dushanzi Petrochemical Company Author: Xi Jun Period: From June, 2014 to April 2016 Country/Region: Gansu Province, P.R.C Network owner: PetroChina Kunlun Natural Gas Tianshui Company Engineer/Installer: Sichuan Hongda Petroleum & Natural Gas Engineering Company Pipe producer: Pipe Producing Division of PetroChina Kunlun Natural Gas Company This installation project is for two newly-build residential communities named Shanshuixincheng and Jinxiuyuan in Qinzhou District of Tianshui City, of which either belong to the Government Affordable Housing programme. In Shanshuixincheng residential community, which lies at Xishili of Qinzhou District and has 3942 apartments, 660 meters of MP pipe and 1130 meters of LP pipe have been installed. In Jinxiuyuan residential community, which has 3982 apartments, 524 meters of MP pipe and 750 meters of LP pipe have been installed. Tianshui City in the meantime owns a low pressure pipeline network of 96 km and 310 km of a medium pressure network. By this more than 60,000 residential consumers, 129 commercial consumers and 2 industrial consumers and one CNG switch station in Qinzhou District, Maiji District and Tianshui National Economic & Technical Development Zone were connected with peak gas supply amount at 50,000 cubic meter per day. The project was realized by PetroChina Kunlun Natural Gas Tianshui company after 8 year of construction. Urban Routing of Natural Gas Network of PetroChina Kunlun Natural Gas Tianshui Company The project of installing gas pipes was realized with diameters ranging from 63 mm to 250 mm. As these communities are designed to use PE100 pipe, no alternative/previous materiall was used. -
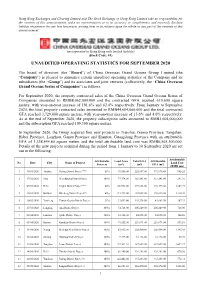
Unaudited Operating Statistics for September 2020
Hong Kong Exchanges and Clearing Limited and The Stock Exchange of Hong Kong Limited take no responsibility for the contents of this announcement, make no representation as to its accuracy or completeness and expressly disclaim liability whatsoever for any loss howsoever arising from or in reliance upon the whole or any part of the contents of this announcement. (incorporated in Hong Kong with limited liability) (Stock Code: 81) UNAUDITED OPERATING STATISTICS FOR SEPTEMBER 2020 The board of directors (the “Board”) of China Overseas Grand Oceans Group Limited (the “Company”) is pleased to announce certain unaudited operating statistics of the Company and its subsidiaries (the “Group”) and its associates and joint ventures (collectively, the “China Overseas Grand Oceans Series of Companies”) as follows: For September 2020, the property contracted sales of the China Overseas Grand Oceans Series of Companies amounted to RMB8,062,000,000 and the contracted GFA reached 610,600 square meters, with year-on-year increase of 101.6% and 62.4% respectively. From January to September 2020, the total property contracted sales amounted to RMB44,604,000,000 and the total contracted GFA reached 3,729,000 square meters, with year-on-year increase of 15.6% and 4.6% respectively. As at the end of September 2020, the property subscription sales amounted to RMB1,604,000,000 and the subscription GFA reached 109,300 square meters. In September 2020, the Group acquired four new projects in Tianshui, Gansu Province, Tangshan, Hebei Province, Lanzhou, Gansu Province and Shantou, Guangdong Province with an attributable GFA of 1,358,444.80 square meters and the total attributable land cost was RMB6,505,300,000. -
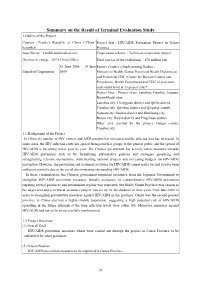
Summary on the Result of Terminal Evaluation Study
Summary on the Result of Terminal Evaluation Study 1.Outline of the Project Country:People’s Republic of China (“China” Project title:HIV/AIDS Prevention Project in Gansu hereafter) Province Issue/Sector:Health and medical care Cooperation scheme:Technical cooperation project Division in charge:JICA China Office Total cost (as of the evaluation):470 million yen 15 June 2006 – 14 June Partner Country’s Implementing Bodies: Period of Cooperation 2009 Ministry of Health, Gansu Provincial Health Department and Provincial CDC (Center for Disease Control and Prevention), Health Department and CDC of prefecture and county level at 13 project sites* Project Sites:Project cities: Lanzhou, Tianshui, Jiuquan, BaiyinModel sites: Lanzhou city: Chengguan district and Qilihe district Tianshui city: Qinzhou district and Qingshui county Jiuquan city: Suzhou district and Dunhuang city Baiyin city: Baiyin district and Pingchuan district Other area assisted by the project: Gangu county, Tianshui city 1.1.Background of the Project In China the number of HIV carriers and AIDS patients has increased and the affected area has increased. In some areas, the HIV infection route has spread from particular groups to the general public and the spread of HIV/AIDS is becoming worse year by year. The Chinese government has actively taken measures towards HIV/AIDS prevention such as by formulating preventative policies and strategies, preparing and strengthening relevant mechanisms, implementing national projects and increasing budgets for HIV/AIDS prevention. However, the prevention and treatment activities for HIV/AIDS cannot really be said to have been sufficient, partially due to the social discrimination surrounding HIV/AIDS. In these circumstances, the Chinese government requested assistance from the Japanese Government to strengthen HIV/AIDS prevention measures. -

Minimum Wage Standards in China August 11, 2020
Minimum Wage Standards in China August 11, 2020 Contents Heilongjiang ................................................................................................................................................. 3 Jilin ............................................................................................................................................................... 3 Liaoning ........................................................................................................................................................ 4 Inner Mongolia Autonomous Region ........................................................................................................... 7 Beijing......................................................................................................................................................... 10 Hebei ........................................................................................................................................................... 11 Henan .......................................................................................................................................................... 13 Shandong .................................................................................................................................................... 14 Shanxi ......................................................................................................................................................... 16 Shaanxi ...................................................................................................................................................... -

20210128 PVR Loan 2760 PRC Gansu Tianshui Urban
Validation Report January 2021 People’s Republic of China: Gansu Tianshui Urban Infrastructure Development Project Reference Number: PVR-758 Project Number: 43025-013 Loan Number: 2760 ABBREVIATIONS ADB – Asian Development Bank CHG – China Huaneng Group CHP – combined heat and power plant CO2 – carbon dioxide DMF – design and monitoring framework EIRR – economic internal rate of return FIRR – financial internal rate of return GTEZ – Guanzhong–Tianshui Economic Zone LAR – land acquisition and resettlement m2 – square meter O&M – operation and maintenance PCR – project completion report PRC – People’s Republic of China THC – Tianshui Heating Company TMG – Tianshui Municipal Government TPMO – Tianshui project management office NOTE In this report, “$” refers to United States dollars. Director General Marvin Taylor-Dormond, Independent Evaluation Department (IED) Deputy Director General Veronique Salze-Lozac’h, IED Director Nathan Subramaniam, Sector and Project Division (IESP) Team Leader Srinivasan Palle Venkata, Senior Evaluation Specialist, IESP The guidelines formally adopted by the Independent Evaluation Department (IED) on avoiding conflict of interest in its independent evaluations were observed in the preparation of this report. To the knowledge of IED management, there were no conflicts of interest of the persons preparing, reviewing, or approving this report. The final ratings are the ratings of IED and may or may not coincide with those originally proposed by the consultants engaged for this report. In preparing any evaluation report, or by making any designation of or reference to a particular territory or geographic area in this document, IED does not intend to make any judgments as to the legal or other status of any territory or area. -

Environmental Assessment Report (DRAFT) People's Republic Of
Environmental Assessment Report (DRAFT) Project Number: 43025 December 2010 People’s Republic of China: Gansu Tianshui Urban Infrastructure Development Project Prepared by Tianshui Municipal Government for the Asian Development Bank (ADB) This environmental impact assessment is a document of the borrower. The views expressed herein do not necessarily represent those of ADB’s Board of Directors, Management, or staff, and may be preliminary in nature. Your attention is directed to the ―Terms of Use‖ section of this website. I CURRENCY EQUIVALENTS (as of 15 October 2010) Currency Unit – Yuan (CNY) CNY1.00 = $0.1503 $1.00 = CNY6.6544 For the purpose of calculations in this report, an exchange rate of $1.00 = CNY6.60 is used. ABBREVIATIONS ADB - Asian Development Bank BOD5 - biochemical oxygen demand in five days CEIA - consolidated environmental impact assessment CHP - combined heat and power CO2 - carbon dioxide CODcr - chemical oxygen demand CSC - construction supervision company EA - executing agency EIA - environmental impact assessment EMP - environmental management plan EMU - environmental management unit FSR - feasibility study report GDP - gross domestic product GEPB - Gansu Environment Protection Bureau GHG - greenhouse gas GRM - grievance redress mechanism HES - heat exchange stations IA - implementing agency IEE - initial environment evaluation LIEC - loan implementation environmental consultants MEP - Ministry of Environmental Protection MFMB - Tianshui Municipal Facilities Management Bureau MLG - minimum living guarantee MV - -

The Preventive Effect and Enhance Immunity Function of Bu-Zhong-Yi-Qi Wan on S180 Tumor Mice
Hu et al., Afr J Tradit Complement Altern Med. (2016) 13(3):60-65 http://dx.doi.org/10.4314/ajtcam.v13i3.8 THE PREVENTIVE EFFECT AND ENHANCE IMMUNITY FUNCTION OF BU-ZHONG-YI-QI WAN ON S180 TUMOR MICE. Fang Hu1,2, Xiao-ya Liu1, Xin-yue Chen1, Can Li1, Rui-juan Zhu1, Huan Han1, Ying-dong Li3, Shi-lan Feng*1 1 School of Pharmacy, Lanzhou University, 199 Donggang west Road, Lanzhou 730000, China. 2 The First Hospital of Tianshui, 105 Jianshe Road Qinzhou District, Tianshui 741000, China. 3Department of Pharmacy, Gansu University of traditional Chinese medicine, 35 Dingxi east Road, Lanzhou 730000, China *Corresponding author: E-mail: [email protected] Abstract Background: To evaluate the preventive effect and enhance immunity functions of Bu-zhong-yi-qi wan in vivo. Materials and Methods: S180 tumor mice model was established by subcutaneous injection a dose of 0.2 ml (1×107/ml) at the right oxter. The inhibitory rates, spleen indexes and thymus indexes were calculated. Splenic lymphocyte proliferative activity assay and phagocytosis activity of peritoneal macrophages were done. Interferon (IFN-γ), interleukin (IL-2) and tumor necrotic factor (TNF-α) in serum were detected. Results: In the S180 tumor-bearing mice, Bu-zhong-yi-qi wan with medium-dose (975 mg/kg, 100 mg/l) had potent preventive effect and anti-tumor effect, macrophage phagocytosis and Con A-stimulated splenocyte proliferation were increased as compared with model control treatment. Bu-zhong-yi-qi wan could take part in the immune response by promoting the proliferation and differentiation of T- cells, increasing the activity of the macrophages, inducing the generation of cell factor IL-2, TNF-α, IFN-γ.