Investigation of Empathy-Like Behavior in Social Housing by Jillian L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download The
ACTIONS OF ISOVALINE AND ENDOGENOUS AMINO ACIDS ON INHIBITORY RECEPTORS IN VENTROBASAL THALAMUS by JAMES EDWARD COOKE BSc, Carleton University, 2002 MSc, Carleton University, 2004 A THESIS SUBMITTED IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in THE FACULTY OF GRADUATE STUDIES (Pharmacology) THE UNIVERSITY OF BRITISH COLUMBIA (Vancouver) August, 2010 © James Edward Cooke, 2010 ABSTRACT This thesis consists of three manuscripts that examine the effects of amino acids and inhibitory neurotransmission in ventrobasal thalamus, a region of the brain responsible for processing nociceptive information. In the first manuscript we examined the possibility that a proposed antagonist of receptors for endogenous amino acids was selective for -amino acids. In the second manuscript, we determined the ionic mechanism of action of isovaline, a non-biogenic amino acid with chemical similarity to glycine and GABA. In the third manuscript, we determined that the inhibitory action of isovaline is mediated by metabotropic receptors, likely GABAB. In the first manuscript we used whole-cell patch clamp electrophysiology and immunohistochemistry to examine the differential antagonism of GABAAergic IPSCs by a proposed -amino acid antagonist, TAG. In IPSCs that were attributable to both GABAergic and glycinergic stimulation, TAG significantly reduced both components. TAG had no effect in purely GABAAergic IPSCs. Our data supports the hypothesis that a specific GABAA subunit, 4, is sensitive to the -amino acid antagonist, TAG. The second manuscript examines the ionic mechanism of action of isovaline, demonstrated to have analgesic properties in animal models. Isovaline inhibited action potential firing of thalamocortical neurons by activating a long-lasting potassium conductance that was insensitive to the glycine antagonist, strychnine. -

1% Showed Wide Receptive Fields, Or Bilat- Eral Representation
Somatotopic Organization and Response Properties of Neurons of the Ventrobasal Complex in the Opossum ' AGLAI P. B. SOUSA,Z E. OSWALDO-CRUZ AND R. GATTASS 2 Departamento de Neurobiologia, Institvto de Bioflsica, 458 av. Pasteur, Rio de Janeiro, Brad ABSTRACT Thalamic responses to somatic stimulation were studied in 47 specimens of the South American opossum, D. marsupialis aurita. Topographic representation of the body surface in the ventrobasal complex was determined by recording unit cluster responses to natural stimulation in sodium pentobarbital anesthetised animals. The pattern of representation observed was similar to that reported in the North American subspecies and comparable to the findings in eutherian mammals. The analysis of single units recorded with extra-cellular microelectrodes showed that the great majority of neurons are place and modality specific. A predominance of skin (vs. deep) sensibility was observed. Units activated by displacement of hairs and vibrissae displayed receptive fields similar to those reported in higher mammals, Of the units isolated within the anatomical bound- aries of the ventrobasal complex only 1% showed wide receptive fields, or bilat- eral representation. None of the units could be activated by auditory stimulation. Neurons displaying wide, discontinuous or bilateral receptive fields showing auditory-somatic convergence were isolated in nucleus paraf ascicularis, subpara- fascicularis and in the Posterior group Using anatomical techniques it has been nervation density are related to a larger shown, in a wide variety of mammals, that voIume of thalamic tissue. components of the ventrobasal complex Single unit recording from this region in (xVB), as defined by Rose and Mount- cats showed that the neurons receiving castle ('52) are the main site of termina- lemniscal projections are place and modal- tion of the medial lemniscus. -

Review Article
Malaysian Journal of Medical Sciences, Vol. 13, No. 2, July 2006 (11-18) REVIEW ARTICLE THE ROLE OF THE THALAMUS IN MODULATING PAIN Che Badariah Ab Aziz & Asma Hayati Ahmad Department of Physiology School of Medical Sciences, Universiti Sains Malaysia 16150 Kubang Kerian, Kelantan, Malaysia The thalamus is one of the structures that receives projections from multiple ascending pain pathways. The structure is not merely a relay centre but is involved in processing nociceptive information before transmitting the information to various parts of the cortex. The thalamic nuclei are involved in the sensory discriminative and affective motivational components of pain. Generally each group of nucleus has prominent functions in one component for example ventrobasal complex in sensory discriminative component and intralaminar nuclei in affective- motivational component. The thalamus is also part of a network that projects to the spinal cord dorsal horn and modulates ascending nociceptive information. In the animal models of neuropathic pain, changes in the biochemistry, gene expression, thalamic blood flow and response properties of thalamic neurons have been shown. These studies suggest the important contribution of the thalamus in modulating pain in normal and neuropathic pain condition. Key words : thalamus, pain, electrophysiology, imaging Submitted-20-02-2004, Accepted-03-12-05 Is there a role for the thalamus in modulating pain? dual system at each level and the sensation of pain that arrives in the central nervous system is The classic pain pathway as was previously composed of the sensory discriminative component understood consists of a three-neuron chain that of pain (first pain), and the affective-motivational transmits pain information from the periphery to the component of pain (second pain), which is carried cerebral cortex (1). -

The Dorsal Column Nuclei Neuroanatomy Reveals a Complex Sensorimotor Integration and Distribution Hub
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 8 November 2019 doi:10.20944/preprints201911.0084.v1 The Dorsal Column Nuclei Neuroanatomy Reveals A Complex Sensorimotor Integration and Distribution Hub Alastair J Loutit1,2, Richard M Vickery1, and Jason R Potas1,2 * 1School of Medical Sciences, UNSW Sydney, Sydney, New South Wales, 2052, Australia 2The Eccles Institute of Neuroscience, John Curtin School of Medical Research, Australian National University, Canberra, Australian Capital Territory, 2601, Australia * Correspondence: Dr Jason R Potas [email protected] Number of words (excluding references, abstract and abbreviations): 14,490 Number of figures: 6 Number of Tables: 1 Number of supplementary figures: 0 Keywords: brainstem sensory nuclei; somatosensation; secondary afferents; posterior column Abstract The dorsal column nuclei (DCN) are organised by both somatotopy and modality, and have a diverse range of afferent inputs and projection targets. The functional organisation and connectivity of the DCN implicate them in a variety of sensorimotor functions, beyond their commonly accepted role in processing and transmitting somatosensory information to the thalamus, yet this is largely underappreciated in the literature. In this review, we examine the morphology, organisation, and connectivity of the DCN and their associated nuclei, to improve understanding of their sensorimotor functions. First, we briefly discuss the receptors, afferent fibres, and pathways involved in conveying tactile and proprioceptive information to the DCN. Next, we review the modality and somatotopic arrangements of the constituents of the dorsal column nuclei complex (DCN-complex), which includes the gracile, cuneate, external cuneate, X, and Z nuclei, and Bischoff’s nucleus. Finally, we examine and discuss the functional implications of the myriad of DCN-complex projection targets throughout the midbrain, and hindbrain, in addition to their modulatory inputs from the cortex. -

The Medial Division of the Medial Geniculate Body of the Cat: Implications for Thalamic Organization’
0270.6474/83/0312-2629$02.00/O The Journal of Neuroscience Copyright 0 Society for Neuroscience Vol. 3, No. 12, pp. 2629-2651 Printed in U.S.A. December 1983 THE MEDIAL DIVISION OF THE MEDIAL GENICULATE BODY OF THE CAT: IMPLICATIONS FOR THALAMIC ORGANIZATION’ JEFFERY A. WINER*,’ AND D. KENT MOREST$ * Department of Physiology-Anatomy, University of California, Berkeley, California 94720 and $ Department of Anatomy, The University of Connecticut Health Center, Farmington, Connecticut 06032 Received September 15,1982; Revised July 29, 1983; Accepted August 2,1983 Abstract The structure of neurons and axons was studied in the medial division of the medial geniculate body of the cat with the Golgi methods. The results show that the medial division consists of morphologically heterogeneous neurons. The main types, in descending order of frequency, are medium-sized neurons with (1) radiate, (2) tufted, or (3) elongate dendrites; (4) small stellate or radiate neurons, including Golgi type II cells with a locally arborizing, sparsely branching axon collateral system; (5) large neurons, which are weakly tufted. A variety of afferent axons impose a reticulate appearance on the fiber architecture of the medial division. The dominant element. in the neuropil consists of axons terminating in the medial division as well as a collateral system of fibers traveling to the adjacent ventral and dorsal divisions of the medial geniculate body. Four types of extrinsic axons are described, including two kinds of thin axons with collateral systems, thick fibers with restricted branches, and large axons with elaborate, serpentine collaterals. Compared to the dorsal and ventral divisions of the medial geniculate body, where, respectively, radiate and tufted neurons are more frequent, the medial division is intermediate in a sense-not that the degree of radiate or tufted dendritic branching is less well developed, but neither type of cell predominates. -

Distributed Processing of Pain and Vibration by the Human Brain
The Journal of Neuroscience, July 1994, 14(7): 4095-4108 Distributed Processing of Pain and Vibration by the Human Brain Robert C. Coghill,’ Jeanne D. Talbot,’ Alan C. Evans,* Ernst Meyer,2 Albert Gjedde,* M. Catherine Bushnell,’ and Gary H. Duncan’ ‘Centre de Recherche en Sciences Neurologiques and Facultk de Mbdecine Dentaire, Universitb de Mont&al, Montreal, Quebec, Canada H3C 3J7 and ‘Positron Imaging Laboratories, McConnell Brain Imaging Center, Montreal Neurological Institute, Montreal, Quebec, Canada H3A 2B4 Pain is a diverse sensory and emotional experience that [Key words: pain, vibration, thalamus, positron emission likely involves activation of numerous regions of the brain. tomography, human, primary somatosensory cortex, insula, Yet, many of these areas are also implicated in the pro- secondary somatosensory cortex, heat, cingulate cortex] cessing of nonpainful somatosensory information. In order to better characterize the processing of pain within the hu- man brain, activation produced by noxious stimuli was com- An understandingof nociceptive processingin the human brain pared with that produced by robust innocuous stimuli. Painful has remained elusive, perhaps becauseof the complexity and heat (47-48”C), nonpainful vibratory (110 Hz), and neutral multiplicity of mechanismssupporting this essential sensory control (34°C) stimuli were applied to the left forearm of right- experience. Early observations by neurosurgeonsled to the view handed male subjects. Activation of regions within the di- that pain is a diencephalic phenomenon and that the telen- encephalon and telencephalon was evaluated by measuring cephalonhas little to do with pain perception (Head and Holmes, regional cerebral blood flow using positron emission tomog- 1911; Penfield and Boldrey, 1937). -
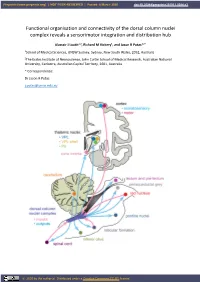
Functional Organisation and Connectivity of the Dorsal Column Nuclei Complex Reveals a Sensorimotor Integration and Distribution Hub
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 8 March 2020 doi:10.20944/preprints201911.0084.v3 Functional organisation and connectivity of the dorsal column nuclei complex reveals a sensorimotor integration and distribution hub Alastair J Loutit1,2, Richard M Vickery1, and Jason R Potas1,2 * 1School of Medical Sciences, UNSW Sydney, Sydney, New South Wales, 2052, Australia 2The Eccles Institute of Neuroscience, John Curtin School of Medical Research, Australian National University, Canberra, Australian Capital Territory, 2601, Australia * Correspondence: Dr Jason R Potas [email protected] © 2020 by the author(s). Distributed under a Creative Commons CC BY license. Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 8 March 2020 doi:10.20944/preprints201911.0084.v3 Abstract The dorsal column nuclei complex (DCN-complex) includes the dorsal column nuclei (DCN, referring to the gracile and cuneate nuclei collectively), external cuneate, X, and Z nuclei, and the median accessory nucleus. The DCN are organised by both somatotopy and modality, and have a diverse range of afferent inputs and projection targets. The functional organisation and connectivity of the DCN implicate them in a variety of sensorimotor functions, beyond their commonly accepted role in processing and transmitting somatosensory information to the thalamus, yet this is largely underappreciated in the literature. To consolidate insights into their sensorimotor functions, this review examines the morphology, organisation, and connectivity of the DCN and their associated nuclei. First, we briefly discuss the receptors, afferent fibres, and pathways involved in conveying tactile and proprioceptive information to the DCN. Next, we review the modality and somatotopic arrangements of the remaining constituents of the DCN-complex. -

Distributions of Spinothalamic, Spinohypothalamic, and Spinotelencephalic Fibers Revealed by Anterograde Transport of PHA-L in Rats
The Journal of Neuroscience, March 1991, 71(3): 652-666 Distributions of Spinothalamic, Spinohypothalamic, and Spinotelencephalic Fibers Revealed by Anterograde Transport of PHA-L in Rats Kenneth D. Cliffer,” Rami Burstein,b and Glenn J. Giesler, Jr. Department of Cell Biology and Neuroanatomy, University of Minnesota, Minneapolis, Minnesota 55455 Fibers projecting from several levels of the spinal cord to they reach these levels (e.g., Lumb and Wolstencroft, 1985; the diencephalon and telencephalon were labeled antero- Werner and Bienek, 1985; Swanson, 1987; Kai et al., 1988; Ma gradely with Phaseolus vulgarisleucoagglutinin injected ion- and Peschan‘ski, 1988). Recently, we reported that cells in the tophoretically. Labeled fibers in the thalamus confirmed pro- spinal cord project directly to the hypothalamus and to several jections previously observed. In addition, many labeled fibers telencephalic areas (Burstein et al., 1987, 1990a; Burstein and were seen in the hypothalamus and in telencephalic areas Giesler, 1989). Cells in the lumbar enlargement of the spinal not generally recognized previously as receiving such pro- cord that project to the hypothalamus respond preferentially to jections. In the hypothalamus, these areas included the lat- noxious input (Burstein et al., 1987). The size of the spinohy- eral hypothalamus (including the medial forebrain bundle), pothalamic tract in rats rivals that of the spinothalamic tract the posterior hypothalamic area, the dorsal hypothalamic (Burstein et al., 1990a,c), a pathway that has been considered area, the dorsomedial nucleus, the pareventricular nucleus, one of the major pathways ascending from the spinal cord to the periventricular area, the suprachiasmatic nucleus, and the brain. Spinotelencephalic pathways appear to be smaller but the lateral and medial preoptic areas. -
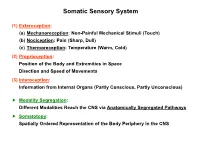
Somatic Sensory System
Somatic Sensory System (1) Exteroception: (a) Mechanoreception: Non-Painful Mechanical Stimuli (Touch) (b) Nociception: Pain (Sharp, Dull) (c) Thermoreception: Temperature (Warm, Cold) (2) Proprioception: Position of the Body and Extremities in Space Direction and Speed of Movements (3) Interoception: Information from Internal Organs (Partly Conscious, Partly Unconscious) Modality Segregation: Different Modalities Reach the CNS via Anatomically Segregated Pathways Somatotopy: Spatially Ordered Representation of the Body Periphery in the CNS Mechanoreception Two-Point Discrimination Mechanoreception Testing the Receptive Fields of Human Sensory Receptors Mechanoreception Variations of Skin Receptors in Receptive Field Size and Adaptation Rate Mechanoreception Merkel's Disk Meissner's Corpuscle Meissner's Corpuscle Masson Trichrome Pacinian Corpuscle Pacinian Corpuscle Masson Trichrome Nociception Free Nerve Endings ! First Pain: Fast, Sharp, Mediated by Myelinated A Fibers Second Pain: Longer-Lasting, Dull, Mediated by Unmyelinated C Fibers Thermoreception Free Nerve Endings ! Cold Receptors: Respond to Temperatures 35C and 45C Warm Receptors: Respond to Temperatures 40C Proprioception = Kinaesthesia Position of the Body and Extremities in Space Direction and Speed of Movements Muscle Spindle dl Detection of: l, l, dt Muscle Spindle Muscle Spindle Alpha Motor Neurons, Gamma Motor Neurons, and the Muscle Fibers they Innervate Muscle Spindle Function of Gamma Motor Neurons Muscle Spindle Azan Knee Jerk Monosynaptic Reflex Golgi Tendon -

Rodent Somatosensory Thalamocortical Circuitry: Neurons, Synapses, and Connectivity
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 5 July 2020 doi:10.20944/preprints202007.0053.v1 Rodent somatosensory thalamocortical circuitry: neurons, synapses, and connectivity 1,2,3 3 4 3,5,6 Christian O’Reilly , Elisabetta Iavarone , Jane Yi & Sean L. Hill 1 Azrieli Centre for Autism Research, Montreal Neurological Institute, McGill University, Montreal, Canada 2 Ronin Institute, Montclair, New Jersey, USA 3 Blue Brain Project, École Polytechnique Fédérale de Lausanne, Geneva, Switzerland 4 Brain Mind Institute, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland 5 Department of Psychiatry, University of Toronto, Toronto, Canada 6 Centre for Addiction and Mental Health, Toronto, Canada Contact author information: Christian O’Reilly, Ph.D. McGill University 3775 Rue University, Room C18 Duff Medical Building Montréal, Québec, H3A 2B4 [email protected] 1-438-933-3696 © 2020 by the author(s). Distributed under a Creative Commons CC BY license. Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 5 July 2020 doi:10.20944/preprints202007.0053.v1 Keywords: thalamocortical loop; thalamus; microcircuit; modeling; reticular nucleus; somatosensory system; ventral posteromedial nucleus; ventral posterolateral nucleus; posterior nucleus; thalamic relay cells; thalamic interneurons; rodent Declarations of interest: none. Highlights: ● We review the circuitry of the somatosensory thalamocortical system in rodents ● We provide an overview of the thalamocortical interdependencies from a modeling perspective ● It provides an accessible yet comprehensive reference on thalamic microcircuitry ● It covers the properties of neurons, synapses, network connectivity, and neuroanatomy ● We identify gaps in current knowledge in order to guide future research Abstract As our understanding of the thalamocortical system deepens, the questions we face become more complex. -

Thalamocortical Connections of Parietal Somatosensory Cortical
Cerebral Cortex September 2009;19:2038--2064 doi:10.1093/cercor/bhn229 Advance Access publication February 16, 2009 Thalamocortical Connections of Parietal Jeffrey Padberg1, Christina Cerkevich2, James Engle1,3, Alexander T. Rajan1, Gregg Recanzone1,4, Jon Kaas2 and Somatosensory Cortical Fields in Leah Krubitzer1,3 Macaque Monkeys are Highly Divergent 1Center for Neuroscience, University of California, Davis, Davis, and Convergent CA, USA, 2Department of Psychology, Vanderbilt University, Nashville, TN 37201, USA, 3Department of Psychology and 4Department of Neurobiology, Physiology & Behavior, University of California, Davis, Davis, CA 95618, USA We examined the organization and cortical projections of the from different receptors combined within and across these Downloaded from https://academic.oup.com/cercor/article/19/9/2038/279997 by guest on 01 October 2021 somatosensory thalamus using multiunit microelectrode recording fields to extract sensory information necessary for texture and techniques in anesthetized monkeys combined with neuroanatom- shape discrimination, object identification and recognition, and ical tracings techniques and architectonic analysis. Different more complex abilities such as generating body or limb portions of the hand representation in area 3b were injected with centered coordinates necessary for intentional reaching and different anatomical tracers in the same animal, or matched body grasping (Randolph and Semmes 1974; Carlson 1981; Schwartz part representations in parietal areas 3a, 3b, 1, 2, and areas 2 and 5 1983; Gardner 1988; Ferraina and Bianchi 1994; Lacquaniti were injected with different anatomical tracers in the same animal et al. 1995; Snyder et al. 1997; Debowy et al. 2001; see Krubitzer to directly compare their thalamocortical connections. We found and Disbrow 2008, for review)? that the somatosensory thalamus is composed of several Previous studies of the connections of these individual representations of cutaneous and deep receptors of the contralat- areas have lead to several important observations that eral body. -

Control of Functional Connectivity in Cerebral Cortex by Basal Ganglia Mediated Synchronization • Pouzzner
4/10/2020 Control of Functional Connectivity in Cerebral Cortex by Basal Ganglia Mediated Synchronization • Pouzzner Control of Functional Connectivity in Cerebral Cortex by Basal Ganglia Mediated Synchronization Daniel Pouzzner, [email protected] Abstract Since the earliest electroencephalography experiments, large scale oscillations have been observed in the mammalian brain. More recently, they have been identified not only in the cerebral cortex and thalamus, but pervasively in the healthy basal ganglia. The basal ganglia mediated synchronization model, introduced here, implicates these oscillations in the combination of cortical association mechanisms with stimulus-response and reinforcement mechanisms in the basal ganglia. In the core mechanism of the model, oscillatory patterns in cortex are selected by and routed through the basal ganglia to the thalamus phase-coherently, then circulated back to widely separated areas of cortex, synchronizing those areas and functionally connecting them. Corticostriatal and striatonigral conduction delays are crucial to this mechanism, and evidence suggests that these delays are unusually long, and unusually varied, in arrangements that might facilitate learning of useful time alignments and associated resonant frequencies. Other structural arrangements in the basal ganglia show further specialization for this role, with convergence in the inputs from cortex, and divergence in many of the return paths to cortex, that systematically reflect corticocortical anatomical connectivity. The basal ganglia