Questions in Animal Behavior
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

La Logica Del Vivente in Maupertuis: Dall’Attrazione Newtoniana Al Determinismo Psicobiologico
La logica del vivente in Maupertuis: dall’attrazione newtoniana al determinismo psicobiologico Federico Focher (Istituto di Genetica Molecolare “Luigi Luca Cavalli Sforza”, CNR, Pavia) Dopo aver rilanciato con successo la teoria epigenetica della generazione, reinterpretata alla luce dell’attrazione newtoniana e delle affinità chimiche (Vénus physique, 1745), Pierre-Louis Moreau de Maupertuis (1698-1759) approfondì le proprie indagini teoretiche nel campo della genesi degli organismi e delle specie nel Système de la Nature (1754). In quest’opera, al fine di conciliare il postulato di oggettività della scienza - che respinge il ricorso alle cause finali - con le evidenti proprietà teleonomiche degli esseri viventi, e riconoscendo nel contempo inadeguati a tale scopo i pur originali principii meccanicistici avanzati nella Vénus physique, Maupertuis propose un’ardita teoria panpsichista del vivente, di ispirazione spinoziana. Postulando quindi la materia viva, dotata di istinto e di sentimento, Maupertuis immaginò che una qualche forma di intelligenza o di memoria psichica, associata alla materia, dirigesse lo sviluppo dei viventi. Alla luce delle recenti scoperte della biologia, l’originale determinismo psicobiologico avanzato da Maupertuis nel Système de la Nature appare una geniale intuizione della logica dei processi genetici ed evolutivi. Keywords: Maupertuis, teleonomia, scienze della vita, storia della biologia, informazione genetica, panpsichismo, Buffon. Introduzione Mezzo secolo dopo la scoperta della legge della gravitazione universale -

Birdobserver17.4 Page183-188 an Honor Without Profit Richard K
AN HONOR WITHOUT PROFIT by Richard K. Walton eponymy n The derivation of a name of a city, country, era, institution, or other place or thing from the name of a person. Gruson in his Words for Birds gives seven categories for the origins of common bird names: appearance (Black-capped Chickadee), eponymy (Henslow’s Sparrow), echoics (Whooping Crane), habitat (Marsh Wren), behavior (woodpecker), food (oystercatcher), and region (California Condor). The second category comprises people and places memorialized in bird names. Many of our most famous ornithologists as well as a fair number of obscure friends and relations have been so honored. A majority of these names were given during the eighteenth and nineteenth centuries, the pioneering era of North American ornithology. While some of these tributes are kept alive in our everyday birding language, others have slipped into oblivion. Recognition or obscurity may ultimately hinge on the names we use for birds. There is no more famous name in the birding culture than that of John James Audubon. His epic The Birds of America was responsible for putting American science, art, and even literature on the international map. This work was created, produced, promoted, and sold largely by Audubon himself. In the years since his death in 1851, the Audubon legend has been the inspiration for a multitude of ornithological pursuits and causes, both professional and amateur. Audubon painted some five hundred birds in Birds of America and described these in his five-volume Ornithological Biographies. Many of the names given by Audubon honored men and women of his era. -

The Spontaneous Generation Controversy (340 BCE–1870 CE)
270 4. Abstraction and Unification ∗ ∗ ∗ “O`uen ˆetes-vous? Que faites-vous? Il faut travailler” (on his death-bed, to his devoted pupils, watching over him). The Spontaneous Generation Controversy (340 BCE–1870 CE) “Omne vivium ex Vivo.” (Latin proverb) Although the theory of spontaneous generation (abiogenesis) can be traced back at least to the Ionian school (600 B.C.), it was Aristotle (384-322 B.C.) who presented the most complete arguments for and the clearest statement of this theory. In his “On the Origin of Animals”, Aristotle states not only that animals originate from other similar animals, but also that living things do arise and always have arisen from lifeless matter. Aristotle’s theory of sponta- neous generation was adopted by the Romans and Neo-Platonic philosophers and, through them, by the early fathers of the Christian Church. With only minor modifications, these philosophers’ ideas on the origin of life, supported by the full force of Christian dogma, dominated the mind of mankind for more that 2000 years. According to this theory, a great variety of organisms could arise from lifeless matter. For example, worms, fireflies, and other insects arose from morning dew or from decaying slime and manure, and earthworms originated from soil, rainwater, and humus. Even higher forms of life could originate spontaneously according to Aristotle. Eels and other kinds of fish came from the wet ooze, sand, slime, and rotting seaweed; frogs and salamanders came from slime. 1846 CE 271 Rather than examining the claims of spontaneous generation more closely, Aristotle’s followers concerned themselves with the production of even more remarkable recipes. -

New Yorkers Had Been Anticipating His Visit for Months. at Columbia
INTRODUCTION ew Yorkers had been anticipating his visit for months. At Columbia University, where French intellectual Henri Bergson (1859–1941) Nwas to give twelve lectures in February 1913, expectations were es- pecially high. When first approached by officials at Columbia, he had asked for a small seminar room where he could directly interact with students and faculty—something that fit both his personality and his speaking style. But Columbia sensed a potential spectacle. They instead put him in the three- hundred-plus-seat lecture theater in Havemeyer Hall. That much attention, Bergson insisted, would make him too nervous to speak in English without notes. Columbia persisted. So, because rhetorical presentation was as impor- tant to him as the words themselves, Bergson delivered his first American lec- ture entirely in French.1 Among the standing-room-only throng of professors and editors were New York journalists and “well-dressed” and “overdressed” women, all fumbling to make sense of Bergson’s “Spiritualité et Liberté” that slushy evening. Between their otherwise dry lines of copy, the reporters’ in- credulity was nearly audible as they recorded how hundreds of New Yorkers strained to hear this “frail, thin, small sized man with sunken cheeks” practi- cally whisper an entire lecture on metaphysics in French.2 That was only a prelude. Bergson’s “Free Will versus Determinism” lec- ture on Tuesday, February 4th—once again delivered in his barely audible French—caused the academic equivalent of a riot. Two thousand people attempted to cram themselves into Havemeyer. Hundreds of hopeful New Yorkers were denied access; long queues of the disappointed snaked around the building and lingered in the slush. -

(Evolution) Prior to Darwin's Origin of Species
Theories of Species Change (Evolution) Prior to Darwin’s Origin of Species Theories of Species Change Prior to Darwin Entangled with: 1. Theories of geological change. 2. Theories of heredity. 3. And theories of ontogenesis, that is, of individual development– embryology. Notions of Species in the Classical Period of Greece Plato: the essence or form of an organism is eternal and unchanging; embodiment only the appearance of that form. Aristotle: the essence or form of an organism is incorporated in the physical body; the only kind of eternity enjoyed is through continued reproduction. Theories of Species Change in the Early Modern Period Descartes: gradual evolution of physical system according to fix laws (Discourse on Method, 1628). Buffon (1707-88) and Linnaeus (1707-78): God created a limited number of species, but through hybridization and impact of environment, new species appear. Kant: evolutionary development from earth possible only if earth already construed as purposive; species change possible but no evidence (Critique of Judgment, 1790). Transformation of one species into another through change of scaling, from D’Arcy Thompson, On Growth and Form (1942). Carus’s illustration of the Richard Owen’s illustration of the archetype archetype, from his On the Nature of Limbs (1849) Georges Cuvier (1769-1832). Portrait by François-André Vincent Adam’s Mammoth, found in Siberia in 18th century; St. Petersburg Natural History Museum Cuvier’s illustration of the Siberian mammoth; he identified it as an extinct species of elephant (1796). Cuvier’s illustration of the “Ohio Animal,” which he named “mastodon.” Megatherium (i.e., “large animal”), discovered in South America, described by Cuvier as an extinct creature similar to the modern sloth. -

On the Trail of Digestion
Middle School Science Eating to Live and Living to Eat Movement of Energy Through Living and Non-living Systems; Student Resource Matter, Its Structure and Changes; From Atoms to the Universe Name On the Trail of Digestion Read the following section on the history of medical science and answer the questions: January 17, 2002 SCoPE SC070104 Page 1 of 6 Middle School Science Eating to Live and Living to Eat Movement of Energy Through Living and Non-living Systems; Student Resource Matter, Its Structure and Changes; From Atoms to the Universe Spallanzani Lazzaro Spallanzani lived in the second half of the 18th Century. You may remember Spallanzani’s experiments on spontaneous generation. His work on digestion is also famous. In Spallanzani’s time, people knew that the stomach was a strong muscle that could grind and mash food. Investigations of bodies told scientists that. But Spallanzani believed that the stomach also held a chemical process. He began his studies using his own falcons. He would feed the falcons small pieces of meat attached firmly to a string, and when the meat had been in the birds stomachs for some time, he would remove it. (You might remember that birds regurgitate quite easily, and some birds feed their babies this way.) Spallanzani believed that digestion was a chemical process. Another noted scientist, Rene Reaumur disagreed. He wrote: "the gastric juice has no more effect out of the living body in dissolving or digesting the food than water, mucilage, milk, or any other bland fluid." Reaumur claimed to have done experiments to see if digestion could take place outside the body as well as in it. -

Behavioral Ecology and Sociobiology in Post-Truth Society
Behav Ecol Sociobiol (2017) 71:76 DOI 10.1007/s00265-017-2303-7 EDITORIAL Behavioral ecology and sociobiology in post-truth society James F. A. Traniello1 & Theo C. M. Bakker2 # Springer-Verlag Berlin Heidelberg 2017 William Whewell (1833)coinedthetermscientist to describe the falsehoods and hoaxes that have virtually become every- experts in the study of natural phenomena. BNature,^ he day occurrences. Although the study of climate change has wrote, Bis a collection of facts governed by laws….To ascer- been the target of most high-profile assaults, skepticism is not tain such laws of nature is the peculiar business of science.^ restricted to atmospheric science or limited to specific research Today, scientists practice in a post-truth society in which trou- agendas. Online journalists attempt to throw truth and reality bling, and often disturbing, attitudes toward science have be- into question and undermine all science by challenging the come commonplace. Science is being marginalized and sup- peer review process. This attack on how ideas are critically pressed (Vernon 2017). Concerns are raised about risks of evaluated, how data are validated, and the nature of proof is human endangerment resulting from the disregard of science insidious. (Gross 2017). Parallels are drawn between the dissemination What should behavioral ecologists do in the present social andgrowthofBfake news^ and the spread of disease environment of denialism? How should we respond to science (Kucharski 2016). Higgins (2016) sounds the alarm: Blosing its relevance as a source of truth^ (Makri 2017)? The BScientists … should be shocked by the idea of post-truth, answer is that we should preach what we practice in a unified … speak up when scientific findings are ignored,… keep voice that is loud and clear. -

Scientific BIOGRAPHY and the CASE of GEORGES CUVIER
Hist. Sci.) xiv (1976), 101-137 1976HisSc..14..101O SCIENTIFic BIOGRAPHY AND THE CASE OF GEORGES CUVIER: WITH A CRITICAL BIBLIOGRAPHY Dorinda OutralU University of Reading The purpose of this introduction is to provide some interpretative tools for the reader of the body of secondary literature on Georges Cuvier which is examined in the attached critical bibliography. Criticism and analysis of existing work is therefore emphasized, and the problems in volved in constructing a positive biography of Cuvier are only briefly examined. Not only strictly biographical studies, but also work on all aspects of Cuvier's achievement, have been so strongly informed by pre suppositions about his character, that a knowledge of this bias and its characteristic expressions is nece.<;sary before previous work on Cuvier can be properly interp'reted. This bibliography is thus also intended as a necessary clearing of the ground before further study of Cuvier's career can be undertaken. This is true not only because it is necessary to discover the precise extent of factual inadequacy in our knowledge of Cuvier's life and achievement, but also because we need to increase our awareness of the role which biographical inquiry has played in the history of science, for without this awareness, the full implications of the adoption of the form cannot be assessed. Interest in Georges Cuvier has increased considerably during the last decade, but so far almost no account has been taken of the extraordinary biographical tradition through which we view him. Almost every presen tation of Cuvier since his death in 1832 has been dominated by emphases which were established very soon afterwards, and which have continued to monopolize the attention of historians of the life-sciences until very recently. -

Copyrighted Material
Chapter 1 History Systematics has its origins in two threads of biological science: classification and evolution. The organization of natural variation into sets, groups, and hierarchies traces its roots to Aristotle and evolution to Darwin. Put simply, systematization of nature can and has progressed in absence of causative theories relying on ideas of “plan of nature,” divine or otherwise. Evolutionists (Darwin, Wallace, and others) proposed a rationale for these patterns. This mixture is the foundation of modern systematics. Originally, systematics was natural history. Today we think of systematics as being a more inclusive term, encompassing field collection, empirical compar- ative biology, and theory. To begin with, however, taxonomy, now known as the process of naming species and higher taxa in a coherent, hypothesis-based, and regular way, and systematics were equivalent. Roman bust of Aristotle (384–322 BCE) 1.1 Aristotle Systematics as classification (or taxonomy) draws its Western origins from Aris- totle1. A student of Plato at the Academy and reputed teacher of Alexander the Great, Aristotle founded the Lyceum in Athens, writing on a broad variety of topics including what we now call biology. To Aristotle, living things (species) came from nature as did other physical classes (e.g. gold or lead). Today, we refer to his classification of living things (Aristotle, 350 BCE) that show simi- larities with the sorts of classifications we create now. In short, there are three featuresCOPYRIGHTED of his methodology that weMATERIAL recognize immediately: it was functional, binary, and empirical. Aristotle’s classification divided animals (his work on plants is lost) using Ibn Rushd (Averroes) functional features as opposed to those of habitat or anatomical differences: “Of (1126–1198) land animals some are furnished with wings, such as birds and bees.” Although he recognized these features as different in aspect, they are identical in use. -
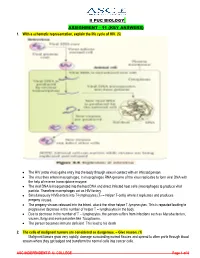
Key Answers) 1
II PUC BIOLOGY ASSIGNMENT – 11 (KEY ANSWERS) 1. With a schematic representation, explain the life cycle of HIV. (5) The HIV (retro virus) gains entry into the body through sexual contact with an infected person. The virus then enters macrophages. In macrophages RNA genome of the virus replicates to form viral DNA with the help of reverse transcriptase enzyme. The viral DNA is incorporated into the host DNA and direct infected host cells (macrophages to produce viral particle. Therefore macrophages act as HIV factory. Simultaneously HIVB enters into T-lymphocytes (TH – Helper T-cells) where it replicates and produces progeny viruses. The progeny viruses released into the blood, attack the other helper T-lymphocytes. This is repeated leading to progressive decrease in the number of helper T – lymphocytes in the body. Due to decrease in the number of T – lymphocytes, the person suffers from infections such as Mycobacterium, viruses, fungi and even parasites like Toxoplasma. The person becomes immune deficient. This lead to his death. 2. The cells of malignant tumors are considered as dangerous. – Give reason. (1) Malignant tumors grow very rapidly, damage surrounding normal tissues and spread to other parts through blood stream where they get lodged and transform the normal cells into cancer cells. ASC INDEPENDENT P. U. COLLEGE Page 1 of 6 3. Explain the salient features of double helix model of DNA. (5) WATSON AND CRICK MODEL OF DNA (Double helix model) * Rosalind Franklin is best known for her work on the X-ray diffraction images (1952) of DNA, which led to the discovery of the DNA double helix model (1953) for which Francis Crick, James Watson, and Maurice Wilkins shared the Nobel Prize in Physiology or Medicine in 1962. -

History of Microbiology: Spontaneous Generation Theory
HISTORY OF MICROBIOLOGY: SPONTANEOUS GENERATION THEORY Microbiology often has been defined as the study of organisms and agents too small to be seen clearly by the unaided eye—that is, the study of microorganisms. Because objects less than about one millimeter in diameter cannot be seen clearly and must be examined with a microscope, microbiology is concerned primarily with organisms and agents this small and smaller. Microbial World Microorganisms are everywhere. Almost every natural surface is colonized by microbes (including our skin). Some microorganisms can live quite happily in boiling hot springs, whereas others form complex microbial communities in frozen sea ice. Most microorganisms are harmless to humans. You swallow millions of microbes every day with no ill effects. In fact, we are dependent on microbes to help us digest our food and to protect our bodies from pathogens. Microbes also keep the biosphere running by carrying out essential functions such as decomposition of dead animals and plants. Microbes are the dominant form of life on planet Earth. More than half the biomass on Earth consists of microorganisms, whereas animals constitute only 15% of the mass of living organisms on Earth. This Microbiology course deals with • How and where they live • Their structure • How they derive food and energy • Functions of soil micro flora • Role in nutrient transformation • Relation with plant • Importance in Industries The microorganisms can be divided into two distinct groups based on the nucleus structure: Prokaryotes – The organism lacking true nucleus (membrane enclosed chromosome and nucleolus) and other organelles like mitochondria, golgi body, entoplasmic reticulum etc. are referred as Prokaryotes. -

Scientific Racism and the Legal Prohibitions Against Miscegenation
Michigan Journal of Race and Law Volume 5 2000 Blood Will Tell: Scientific Racism and the Legal Prohibitions Against Miscegenation Keith E. Sealing John Marshall Law School Follow this and additional works at: https://repository.law.umich.edu/mjrl Part of the Civil Rights and Discrimination Commons, Law and Race Commons, Legal History Commons, and the Religion Law Commons Recommended Citation Keith E. Sealing, Blood Will Tell: Scientific Racism and the Legal Prohibitions Against Miscegenation, 5 MICH. J. RACE & L. 559 (2000). Available at: https://repository.law.umich.edu/mjrl/vol5/iss2/1 This Article is brought to you for free and open access by the Journals at University of Michigan Law School Scholarship Repository. It has been accepted for inclusion in Michigan Journal of Race and Law by an authorized editor of University of Michigan Law School Scholarship Repository. For more information, please contact [email protected]. BLOOD WILL TELL: SCIENTIFIC RACISM AND THE LEGAL PROHIBITIONS AGAINST MISCEGENATION Keith E. Sealing* INTRODUCTION .......................................................................... 560 I. THE PARADIGM ............................................................................ 565 A. The Conceptual Framework ................................ 565 B. The Legal Argument ........................................................... 569 C. Because The Bible Tells Me So .............................................. 571 D. The Concept of "Race". ...................................................... 574 II.