Socio-Demographic, Not Environmental, Risk Factors Explain
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Endemic Chromoblastomycosis Caused Predominantly by Fonsecaea Nubica, Madagascar
Article DOI: https://doi.org/10.3201/eid2606.191498 Endemic Chromoblastomycosis Caused Predominantly by Fonsecaea nubica, Madagascar Appendix Appendix Table. Characteristics of clinical isolates from patients with suspected chromoblastomycosis in Madagascar and reference strains used in this study* Panfugal PCR for S/C Specific PCR for S/C MALDI-ToF GenBank accession no. Ccar- Code Species for D1D2, ITS Source Origin NL-1/NL-4 ITS1/ITS4 F/Ccar-R Fon-F/Fon-R MSP ID MYC04002 Unidentified NA, NA Biopsy Vatovavy Fitovinany N/P P/N N/N N/NA NA NA MYC04004 Fonsecaea sp. NA, NA Biopsy Vatovavy Fitovinany P/P P/NA N/NA N/P NA NA MYC08006 Fonsecaea sp. MK828325, NA Biopsy Analanjirofo P/P P/NA N/N N/P NA NA MYC10010 Fonsecaea sp. NA, NA Biopsy Melaky P/P P/NA N/NA P/P NA NA MYC10011 Fonsecaea sp. NA, NA Squama Vatovavy Fitovinany P/P P/NA N/NA P/P NA NA MYC10014 Cladophialophora carrionii MK828362, MK820046 Squama Androy P/P P/P N/P N/N X NA MYC03022 Unidentified NA, NA Biopsy Sofia NA/NA NA/NA N/NA NA/NA NA NA MYC04026 Fonsecaea nubica MK828326, MK828125 Biopsy Alaotra Mangoro N/P N/P NA/N NA/P NA X MYC05032 F. nubica MK828327, MK828124 Squama Amoron I Mania N/P N/P NA/N NA/P NA NA MYC06033 Unidentified NA, NA Biopsy Androy N/P N/P NA/N NA/N NA NA MYC08040 C. carrionii MK828363, MK820047 Biopsy Atsimo Andrefana N/P N/P NA/P NA/N NA NA MYC09041 Unidentified NA, NA Biopsy Analamanga NA/NA NA/NA NA/NA NA NA NA MYC03048 Unidentified NA, NA Biopsy Vakinankaratra N/P N/N N/N N/N NA NA MYC06057 Unidentified NA, NA Biopsy Amoron I Mania P/P N/N N/N N/N NA NA MYC06059 F. -
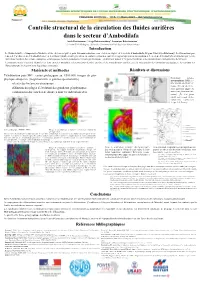
Contrôle Structural De La Circulation Des Fluides Aurifères Dans Le
Contrôle structural de la circulation des fluides aurifères dans le secteur d’Ambodilafa Anick Ratefiarimino 1, Serge Rakotonanahary1, Dominique Rakotomanana1 1 : Mention Génie Géologique (Université d’Antananarivo-Ecole Supérieure Polytechnique) Introduction Le District aurifère d’Ampasary—Vohilava est une des aires réputées pour leur minéralisation en or et au sein duquel est le secteur d’Ambodilafa (Région Vatovavy—Fitovinany). Les formations por- teuses de l’or du secteur d’Ambodilafa sont en des filons hydrothermaux généralement conformes dans des contextes magmatiques ou métamorphiques. Le secteur d’Ambodilafa est structuré par les dé- formations résultant des actions conjuguées et juxtaposées de deux événements majeurs gondwanéens : cisaillement majeur d’Angavo-Ifanadiana et les déformations curvi-planaires de Maroala. Les travaux menés visaient à identifier les liens entre les modalités et les processus de mise en place de la minéralisation aurifère avec la structuration des formations géologiques ; les conduits des fluides pouvant être façonnés par la géologie structurale. Matériels et méthodes Résultats et discussions Télédétection puis SIG : cartes géologiques au 1/500.000, images de géo- Evénement tectono- physique aéroportée (magnétométrie et gamma-spectrométrie) métamorphique DM1 : re- relevés des linéaments structuraux prise des plis antérieurs ver- ticaux (F1) par un événe- définition des plages d’évolution des grandeurs géophysiques ment postérieur auquel est associé un plissement sub- combinaison des couches de données dont les indications d’or vertical F2. Les plans axiaux sont sécants. Défor- mation finie : replissement de type 2 de Ramsay Carte géologique (PGRM, 2008) Image de géophysique aéroportée (ci-dessus): champ ma gnétique total. Lors de leur décantation les minéraux ont été orientés en fonction des caractères du champ magnétique terrestre régnant. -

Rep 2 out Public 2010 S Tlet Sur of Ma Urvey Rvey Adagas Repor Scar Rt
Evidence for Malaria Medicines Policy Outlet Survey Republic of Madagascar 2010 Survey Report MINSTERE DE LA SANTE PUBLIQUE www. ACTwatch.info Copyright © 2010 Population Services International (PSI). All rights reserved. Acknowledgements ACTwatch is funded by the Bill and Melinda Gates Foundation. This study was implemented by Population Services International (PSI). ACTwatch’s Advisory Committee: Mr. Suprotik Basu Advisor to the UN Secretary General's Special Envoy for Malaria Mr. Rik Bosman Supply Chain Expert, Former Senior Vice President, Unilever Ms. Renia Coghlan Global Access Associate Director, Medicines for Malaria Venture (MMV) Dr. Thom Eisele Assistant Professor, Tulane University Mr. Louis Da Gama Malaria Advocacy & Communications Director, Global Health Advocates Dr. Paul Lavani Executive Director, RaPID Pharmacovigilance Program Dr. Ramanan Senior Fellow, Resources for the Future Dr. Matthew Lynch Project Director, VOICES, Johns Hopkins University Centre for Dr. Bernard Nahlen Deputy Coordinator, President's Malaria Initiative (PMI) Dr. Jayesh M. Pandit Head, Pharmacovigilance Department, Pharmacy and Poisons Board‐Kenya Dr. Melanie Renshaw Advisor to the UN Secretary General's Special Envoy for Malaria Mr. Oliver Sabot Vice‐President, Vaccines Clinton Foundation Ms. Rima Shretta Senior Program Associate, Strengthening Pharmaceutical Systems Dr. Rick Steketee Science Director, Malaria Control and Evaluation Partnership in Africa Dr. Warren Stevens Health Economist Dr. Gladys Tetteh CDC Resident Advisor, President’s Malaria -

RAPPORT D'activité 2015-2016 Projet D'adaptation De La Gestion Des Zones Côtières Au Changement Climatique
17' 0( (/ 1( ¶( 1 & 2 2 5 / , 2 9 * 1 , ( ( ¶ / ( 7 ( ' ' ( ( 6 5 ) ( 2 7 6 5 , ( 1 , 7 6 0 MINISTERE DE L’ENVIRONNEMENT, DE L’ECOLOGIE ET DES FORETS SECRETARIAT GENERAL BUREAU NATIONAL DE COORDINATION DES CHANGEMENTS CLIMATIQUES RAPPORT D'ACTIVITÉ 2015-2016 Projet d'Adaptation de la gestion des zones côtières au changement climatique PROJET D’AdaptatioN DE LA GESTION DES ZONES CÔTIÈRES AU CHANGEMENT CLIMatiQUE Etant un pays insulaire, Madagascar est Plusieurs actions ont été entreprises par le considéré comme l’un des pays les plus projet d’Adaptation de la gestion des Zones SOMMAIRE vulnérables à la variabilité et aux changements Côtières au changement climatique en tenant climatiques. Les dits changements se compte de l’Amélioration des écosystèmes CONTEXTE 5 manifestent surtout par le «chamboulement et des moyens de subsistance » au cours du régime des pluviométries, l’augmentation de l’année 2016 comme la réalisation des COMPOSANTE 1 : RENForcement DES capacITÉS de la température, la montée du niveau de études de vulnérabilité dans les quatre zones INSTITUTIONNELLES AUX Impacts DU CHANGEMENT la mer et l’intensification des évènements d’intervention, la création d’un mécanisme de CLImatIQUE DANS LES SITES DU proJET climatiques extrêmes tels que les cyclones, les coordination et la mise en place de la Gestion (MENABE, BOENY, VatovavY FItovINANY ET ATSINANANA) 7 inondations et les sècheresses. Devant cette Intégrée des zones côtières dans les régions situation alarmante, des actions d’adaptation Atsinanana, Boeny, et Vatovavy Fitovinany, ainsi COMPOSANTE 2 : RÉHABILItatION ET GESTION DES ZONES sont déja mises en oeuvre à Madagascar afin de que la mise en œuvre des scénarios climatiques CÔTIÈRES EN VUE d’uNE RÉSILIENCE À LONG TERME 17 renforcer la résilience de la population locale et à l’échelle réduite de ces quatre régions. -

Universite D'antananarivo
UNIVERSITE D’ANTANANARIVO FACULTE DES SCIENCES DEPARTEMENT DE PHYSIQUE MEMOIRE Pour l’obtention du diplôme de MAITRISE DES SCIENCES ET TECHNIQUES EN GEOPHYSIQUE APPLIQUEE Option : Mines et Environnement Intitulé ETUDE DES ZONES FAVORABLES EN MINERALISATION AURIFERE ET EN EMERAUDE DANS LA COMMUNE RURALE D’ANDRORANGAVOLA, DISTRICT D’IFANADIANA, REGION VATOVAVY FITOVINANY Présenté par RANTOSOA Andriharizafy Devant la commission d’examen composée de : Président : RANDRIAMANANTANY Zely Arivelo Professeur Titulaire Rapporteur : RASOLOMANANA Eddy Professeur Examinateurs: RANDRIANJA Roger Professeur RAZAFINDRAKOTO Boni Gauthier Docteur Le 29 Décembre 2008 UNIVERSITE D’ANTANANARIVO FACULTE DES SCIENCES DEPARTEMENT DE PHYSIQUE MEMOIRE Pour l’obtention du diplôme de MAITRISE DES SCIENCES ET TECHNIQUES EN GEOPHYSIQUE APPLIQUEE Option : Mines et Environnement Intitulé ETUDE DES ZONES FAVORABLES EN MINERALISATION AURIFERE ET EN EMERAUDE DANS LA COMMUNE RURALE D’ANDRORANGAVOLA, DISTRICT D’IFANADIANA, REGION VATOVAVY FITOVINANY Présenté par RANTOSOA Andriharizafy Devant la commission d’examen composée de : Président : RANDRIAMANANTANY Zely Arivelo Professeur Titulaire Rapporteur : RASOLOMANANA Eddy Professeur Examinateurs: RANDRIANJA Roger Professeur RAZAFINDRAKOTO Boni Gauthier Docteur Le 29 Décembre 2008 REMERCIEMENTS En préambule de ce mémoire, je souhaite adresser ici mes vifs remerciements à toutes personnes qui, de près ou de loin, ont contribué à l'élaboration de ce mémoire tout particulièrement les personnes citées ci après : Madame RANDRIAMANANTANY Zely Arivelo , Professeur Titulaire, Chef du Département de Physique, qui a bien voulu présidé le membre de jury. Monsieur le Professeur RANAIVO Nomenjanahary Flavien , Directeur de l’ Institut et Observatoire de Géophysique d’Antananarivo (IOGA), Responsable Pédagogique de la formation en Maîtrise des Science et Technique en Géophysique Appliqué (MSTGA), et qui m’a accepté d’être parmi ses étudiants au sein dudit établissement. -

626 Votants: 263 Blancs Et Nuls: 3 Suffrage Exprimes: 260
RESULTAT LEGISLATIVES 27 MAI 2019 District: IFANADIANA Commune: AMBOHIMANGA SUD Code Bureau: 350101010101 AMBOHIMANGA SUD EPP AMBOHIMANGA SUD INSCRITS: 626 VOTANTS: 263 BLANCS ET NULS: 3 SUFFRAGE EXPRIMES: 260 N° Partie Voix Poucentage 1 IRD 123 47,31% 2 MAFI 6 2,31% 3 TIM 128 49,23% 4 INDEPENDANT RABEMIAFARA MANARINTSOA JOSÉ 3 1,15% Total des voix 260 RESULTAT LEGISLATIVES 27 MAI 2019 District: IFANADIANA Commune: AMBOHIMANGA SUD Code Bureau: 350101020101 SAHAMARANA EPP SAHAMARANA INSCRITS: 359 VOTANTS: 86 BLANCS ET NULS: 4 SUFFRAGE EXPRIMES: 82 N° Partie Voix Poucentage 1 IRD 51 62,20% 2 MAFI 3 3,66% 3 TIM 27 32,93% 4 INDEPENDANT RABEMIAFARA MANARINTSOA JOSÉ 1 1,22% Total des voix 82 RESULTAT LEGISLATIVES 27 MAI 2019 District: IFANADIANA Commune: AMBOHIMANGA SUD Code Bureau: 350101030101 MANDROALINA EPP MANDROALINA INSCRITS: 302 VOTANTS: 103 BLANCS ET NULS: 2 SUFFRAGE EXPRIMES: 101 N° Partie Voix Poucentage 1 IRD 71 70,30% 2 MAFI 1 0,99% 3 TIM 22 21,78% 4 INDEPENDANT RABEMIAFARA MANARINTSOA JOSÉ 7 6,93% Total des voix 101 RESULTAT LEGISLATIVES 27 MAI 2019 District: IFANADIANA Commune: AMBOHIMANGA SUD Code Bureau: 350101040101 AMBALAHERANA EPP AMBALAHERANA INSCRITS: 176 VOTANTS: 118 BLANCS ET NULS: 0 SUFFRAGE EXPRIMES: 118 N° Partie Voix Poucentage 1 IRD 87 73,73% 2 MAFI 1 0,85% 3 TIM 30 25,42% 4 INDEPENDANT RABEMIAFARA MANARINTSOA JOSÉ 0 0,00% Total des voix 118 RESULTAT LEGISLATIVES 27 MAI 2019 District: IFANADIANA Commune: AMBOHIMANGA SUD Code Bureau: 350101050101 AMBALAVARY EPP AMBALAVARY INSCRITS: 166 VOTANTS: 120 BLANCS ET NULS: -

Download Final Performance Evaluation of the Fararano
Final Performance Evaluation of the Fararano Development Food Security Activity in Madagascar March 2020 |Volume I IMPEL | Implementer-Led Evaluation & Learning Associate Award ABOUT IMPEL The Implementer-led Evaluation & Learning Associate Award works to improve the design and implementation of Food for Peace (FFP) funded development food security activities (DFSAs) through implementer-led evaluations and knowledge sharing. Funded by the USAID Office of Food for Peace (FFP), the Implementer-led Evaluation & Learning Associate Award will gather information and knowledge in order to measure performance of DFSAs, strengthen accountability, and improve guidance and policy. This information will help the food security community of practice and USAID to design projects and modify existing projects in ways that bolster performance, efficiency and effectiveness. The Implementer-led Evaluation & Learning Associate Award is a two-year activity (2019-2021) implemented by Save the Children (lead), TANGO International, and Tulane University in Haiti, the Democratic Republic of Congo, Madagascar, Malawi, Nepal, and Zimbabwe. RECOMMENDED CITATION IMPEL. (2020). Final Performance Evaluation of the Fararano Development Food Security Activity in Madagascar (Vol. 1). Washington, DC: The Implementer-Led Evaluation & Learning Associate Award PHOTO CREDITS Three-year-old child, at home in Mangily village (Toliara II District), after recovering from moderate acute malnutrition thanks to support from the Fararano Project. Photo by Heidi Yanulis for CRS. DISCLAIMER This report is made possible by the generous support of the American people through the United States Agency for International Development (USAID). The contents are the responsibility of the Implementer-Led Evaluation & Learning (IMPEL) award and do not necessarily reflect the views of USAID or the United States Government. -

Le Developpement Economique De La Region Vatovavy Fitovinany
UNIVERSITE D’ANTANANARIVO Année Universitaire : 2006-2007 Faculté de Droit, d’Economie, de Second Cycle – Promotion Sortante Gestion et de Sociologie Option : DEVELOPPEMENT DEPARTEMENT ECONOMIE « Promotion ANDRAINA » Mémoire de fin de Cycle LE DEVELOPPEMENT ECONOMIQUE DE LA REGION VATOVAVY FITOVINANY Encadré par : Monsieur Gédéon RAJAONSON Présenté par : MANIRISOA RAZAFIMARINTSARA Firmin Date de soutenance : 14 Décembre 2007 REMERCIEMENTS Pour commencer, je tiens à exprimer toute ma reconnaissance à tous ceux qui ont contribué, de près ou de loin, à ma formation et à la réalisation de ce Grand Mémoire de fin d’études en Economie. J’adresse donc tout particulièrement mes vifs remerciement à : • DIEU TOUT PUISSANT • Mon encadreur Monsieur Gédéon RAJAONSON ; • Tous les enseignants et les Personnels administratifs du Département Economie de la Faculté DEGS de l’Université d’Antananarivo ; • Monsieur Le Chef de Région de Vatovavy Fitovinany et ses équipes • Monsieur le Directeur Régional des Travaux Publics de Vatovavy Fitovinany • Ma famille pour leurs soutiens permanents. Veuillez accepter le témoignage de ma profonde gratitude. LISTE DES ABREVIATIONS ANGAP : Agence Nationale de la Gestion des Aires Protégés CEG : Collège d’Enseignement Général CHD 1 : Centre Hospitalier de District Niveau 1 CHD 2 : Centre Hospitalier de District Niveau 2 CISCO : Circonscription Scolaire CSB 1 : Centre de Santé de Base Niveau 1 CSB 2 : Centre de Santé de Base Niveau 2 DRDR : Direction Régionale du Développement Rural EPP : Ecole Primaire Public FCE : Fianarantsoa Côte Est FER : Fonds d’Entretien Routier FTM : Foibe Toantsritanin’i Madagasikara GU : Guichet Unique HIMO : Haute Intensité de Main d’œuvre INSTAT : Institut National de la Statistique M.A.E.P. -

Final Performance Evaluation of the Fararano Development Food Security Activity in Madagascar
Final Performance Evaluation of the Fararano Development Food Security Activity in Madagascar March 2020 |Volume II – Annexes J, K, L IMPEL | Implementer-Led Evaluation & Learning Associate Award ABOUT IMPEL The Implementer-Led Evaluation & Learning Associate Award works to improve the design and implementation of Food for Peace (FFP)-funded development food security activities (DFSAs) through implementer-led evaluations and knowledge sharing. Funded by the USAID Office of Food for Peace (FFP), the Implementer-Led Evaluation & Learning Associate Award will gather information and knowledge in order to measure performance of DFSAs, strengthen accountability, and improve guidance and policy. This information will help the food security community of practice and USAID to design projects and modify existing projects in ways that bolster performance, efficiency and effectiveness. The Implementer-Led Evaluation & Learning Associate Award is a two-year activity (2019-2021) implemented by Save the Children (lead), TANGO International, and Tulane University in Haiti, the Democratic Republic of Congo, Madagascar, Malawi, Nepal, and Zimbabwe. RECOMMENDED CITATION IMPEL. (2020). Final Performance Evaluation of the Fararano Development Food Security Activity in Madagascar (Vol. 2). Washington, DC: The Implementer-Led Evaluation & Learning Associate Award PHOTO CREDITS Three-year-old child, at home in Mangily village (Toliara II District), after recovering from moderate acute malnutrition thanks to support from the Fararano Project. Photo by Heidi Yanulis for CRS. DISCLAIMER This report is made possible by the generous support of the American people through the United States Agency for International Development (USAID). The contents are the responsibility of the Implementer-Led Evaluation & Learning (IMPEL) award and do not necessarily reflect the views of USAID or the United States Government. -

Crustal Assembly of the Antananarivo and Masora Domains, Central
Journal of Mineralogical and Petrological Sciences, Volume 110, page 111–125, 2015 Crustal assembly of the Antananarivo and Masora domains, central–eastern Madagascar: constraints from U–Pb zircon geochronology and whole–rock geochemistry of meta–granitoids Takashi ICHIKI*, Masahiro ISHIKAWA*, Jun–Ichi KIMURA**, Ryoko SENDA** and Raymond RAKOTONDRAZAFY*** *Graduate School of Environment and Information Sciences, Yokohama National University, 79–7 Tokiwadai, Hodogaya–ku Yokohama 240–8501, Japan **Department of Solid Earth Geochemistry (D–SEG), Japan Agency for Marine–Earth Science and Technology (JAMSTEC), 2–15 Natsushima–cho, Yokosuka 237–0061, Japan ***Department of Earth Sciences, Faculty of Science, University of Antananarivo, PB 906 Antananarivo 101, Madagascar In reconstructions of the Gondwana supercontinent, correlations of Archean domains between Madagascar and India remain debated. In this paper, we aim to establish correlations among these Archean domains using whole–rock geochemistry and U–Pb zircon geochronology of meta–granitoids from the Masora and the Anta- nanarivo domains, central–eastern Madagascar. A meta–granitoid from the central part of Masora domain is dated at 3277 Ma and shows a typical Archean tonalite–trondhjemite–granodiorite composition, whereas a tonalitic gneiss from the southeastern part of the Antananarivo domain gives an age of 2744 Ma. The geo- chemical signature of this tonalitic gneiss differs from that of the ~ 2500 Ma granitoids of the northwestern part of Antananarivo domain. In addition, the geochemical composition of the ~ 760 Ma granitic gneisses is con- sistent with a volcanic–arc origin for the protolith. Based on the geochemical and geochronological results, along with existing data, we identified three episodes of granitic magmatism in central–eastern Madagascar at ~ 3300, 2700, and 2500 Ma. -

Table Des Matieres
TABLE DES MATIERES LA REGION………………………………………………………………. ………………….1 1 MILIEU PHYSIQUE ...................................................................................................................... 4 1.1 RELIEF ET PAYSAGE ................................................................................................................. 4 1.2 GEOLOGIE ................................................................................................................................... 4 1.3 CLIMAT ........................................................................................................................................ 4 1.1.1 Le réseau de stations météorologiques ........................................................................................... 5 1.1.2 Température ................................................................................................................................... 5 1.1.3 Pluviométrie ................................................................................................................................... 5 1.1.4 Diagramme ombrothermique ......................................................................................................... 7 1.1.5 Vents ............................................................................................................................................... 8 1.1.6 Cyclones ......................................................................................................................................... 9 1.4 HYDROLOGIE ............................................................................................................................ -

The Pmi Vectorlink Madagascar 2018 End of Spray Report (Eosr)
THE PMI VECTORLINK MADAGASCAR 2018 END OF SPRAY REPORT (EOSR) SUBMITTED NOVEMBER 30, 2018 Recommended Citation: The PMI VectorLink Project. November 2018. PMI VectorLink Madagascar 2018 End of Spray Report. Rockville, MD. The PMI VectorLink Project, Abt Associates Inc. Contract Number: AID-OAA-I-17-00008 Task Order Number: AID-OAA-TO-17-00027 Submitted to: United States Agency for International Development/PMI The views expressed in this document do not necessarily reflect the views of the United States Agency for International Development or the United States Government. Abt Associates Inc. | 6130 Executive Boulevard | Rockville, MD 20852 | T. 301.347.5000 | F. 301.913.9061 | www.abtassociates.com THE PMI VECTORLINK MADAGASCAR 2018 END OF SPRAY REPORT (EOSR) Contents Acronyms…. ................................................................................................................................viii Executive Summary ..................................................................................................................... ix 1. Introduction .......................................................................................................................... 11 1.1 Background of IRS in Madagascar ................................................................................................................. 11 1.2 2018 Campaign Objectives ............................................................................................................................. 12 2. Pre-Spray Activities ............................................................................................................