Inner Ear in Balance and Equilibrium
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
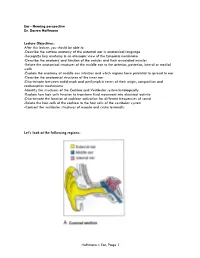
Ear, Page 1 Lecture Outline
Ear - Hearing perspective Dr. Darren Hoffmann Lecture Objectives: After this lecture, you should be able to: -Describe the surface anatomy of the external ear in anatomical language -Recognize key anatomy in an otoscopic view of the tympanic membrane -Describe the anatomy and function of the ossicles and their associated muscles -Relate the anatomical structures of the middle ear to the anterior, posterior, lateral or medial walls -Explain the anatomy of middle ear infection and which regions have potential to spread to ear -Describe the anatomical structures of the inner ear -Discriminate between endolymph and perilymph in terms of their origin, composition and reabsorption mechanisms -Identify the structures of the Cochlea and Vestibular system histologically -Explain how hair cells function to transform fluid movement into electrical activity -Discriminate the location of cochlear activation for different frequencies of sound -Relate the hair cells of the cochlea to the hair cells of the vestibular system -Contrast the vestibular structures of macula and crista terminalis Let’s look at the following regions: Hoffmann – Ear, Page 1 Lecture Outline: C1. External Ear Function: Amplification of Sound waves Parts Auricle Visible part of external ear (pinna) Helix – large outer rim Tragus – tab anterior to external auditory meatus External auditory meatus Auditory Canal/External Auditory Meatus Leads from Auricle to Tympanic membrane Starts cartilaginous, becomes bony as it enters petrous part of temporal bone Earwax (Cerumen) Complex mixture -

Mathematical Model of the Cupula-Endolymph System with Morphological Parameters for the Axolotl (Ambystoma Tigrinum) Semicircular Canals
138 The Open Medical Informatics Journal, 2008, 2, 138-148 Open Access Mathematical Model of the Cupula-Endolymph System with Morphological Parameters for the Axolotl (Ambystoma tigrinum) Semicircular Canals Rosario Vega1, Vladimir V. Alexandrov2,3, Tamara B. Alexandrova1,3 and Enrique Soto*,1 1Instituto de Fisiología, Universidad Autónoma de Puebla, 2Facultad de Ciencias Físico Matemáticas, Universidad Autónoma de Puebla, 3 Lomonosov Moscow State University, Mexico Abstract: By combining mathematical methods with the morphological analysis of the semicircular canals of the axolotl (Ambystoma tigrinum), a system of differential equations describing the mechanical coupling in the semicircular canals was obtained. The coefficients of this system have an explicit physiological meaning that allows for the introduction of morphological and dynamical parameters directly into the differential equations. The cupula of the semicircular canals was modeled both as a piston and as a membrane (diaphragm like), and the duct canals as toroids with two main regions: i) the semicircular canal duct and, ii) a larger diameter region corresponding to the ampulla and the utricle. The endolymph motion was described by the Navier-Stokes equations. The analysis of the model demonstrated that cupular behavior dynamics under periodic stimulation is equivalent in both the piston and the membrane cupular models, thus a general model in which the detailed cupular structure is not relevant was derived. Keywords: Inner ear, vestibular, hair cell, transduction, sensory coding, physiology. 1. INTRODUCTION linear acceleration detectors, and the SCs as angular accel- eration detectors, notwithstanding that both sensory organs The processing of sensory information in the semicircular are based on a very similar sensory cell type. -

Organum Vestibulocochleare INTERNAL EAR MIDDLE EAR EXTERNAL EAR PETROSAL BONE- Eq EXTERNAL EAR AURICLE
EAR organum vestibulocochleare INTERNAL EAR MIDDLE EAR EXTERNAL EAR PETROSAL BONE- Eq EXTERNAL EAR AURICLE The external ear plays the role of an acoustic antenna: auricle the auricle (together with the head) collects and focuses sound waves, the ear canal act as a resonator. tympanic membrane anular cartilage meatus acusticus externus EXTERNAL EAR EXTERNAL EAR AURICLE scutiform cartilage Auricular muscles: -Dorsal -Ventral -Rostral -Caudal EXTERNAL EAR MEATUS ACUSTICUS EXTERNUS auricular cartilage vertical canal auditory ossicles horizontal cochlea canal auditory tube tympanic tympanic eardrum bulla cavity tympanic membrane MIDDLE EAR Auditory ossicles STAPES INCUS Tympanic cavity: (anvil) (stirrup) - epitympanium - mesotympanium - hypotympanium MALLEUS (hammer) auditory vestibular window- ossicles or oval window through which mechanical stimuli (transmitted by the auditory ossicles) enter the epitympanic internal ear for translation recess into nerve impulses auditory tube (Eustachian tube) cochlear window- or round window tympanic cavity bulla tympanica through which the vibration of the perilympha is absorbed MIDDLE EAR MIDDLE EAR GUTTURAL POUCH- Eq MIDDLE EAR AUDITORY OSSICLES head INCUS processus rostralis (stirrup) STAPES processus muscularis (anvil) manubrium short crus body MALLEUS (hammer) Two muscles of the ossicles: long crus m. tensor tympani- n. tensoris tympani ex. n. base mandibularis (footplate) m. stapedius- n. stapedius ex. n. facialis crus The muscles fix the bones and protect the cochlea crus against the harmful effects -

Tip-Link Integrity and Mechanical Transduction in Vertebrate Hair Cells
Neuron, Vol. 7, 985-994, December, 1991, Copyright 0 1991 by Cell Press Tip-link Integrity and Mechanical Transduction in Vertebrate Hair Cells John A. Assad,*+ Gordon M. G. Shepherd,* This suggests that the gating springs are not rigid ele- and David P. Corey*511 ments, but can be slack-that they can pull but not *Department of Neurobiology push on the channels (Corey and Hudspeth, 1983). *Program in Neuroscience The structural correlate of this process has not been Harvard Medical School well established. A simple model has evolved from Boston, Massachusetts 02115 several independent observations. First, measure- SDepartment of Neurology ment of current flow near moving bundles indicated Massachusetts General Hospital thatthetransductionchannelsareatornearthetipsof Boston, Massachusetts 02114 the stereocilia (Hudspeth, 1982). While this has been IINeuroscience Group challenged by measurements with a Ca*+ indicator Howard Hughes Medical Institute dye (Ohmori, 1988), two additional experirnents have corroborated the localization of the channels at the tips (Huang and Corey, 1990, Biophys. Sot., abstract; Summary Jaramillo and Hudspeth, 1991). Second, the discovery of fine filaments between the tips of adjacent ster- An attractive hypothesis for hair-cell transduction is that eocilia led to the suggestion that these “tip links”were fine, filamentous “tip links” pull directly on mechani- the actual mechanical linkages to the channels (Pick- cally sensitive ion channels located at the tips of the les et al., 1984). The geometry of the bundle is such stereocilia. We tested the involvement of tip links in the that excitatory displacements would stretch the tip transduction process by treating bundles with a BAPTA- links and apply tension to the channels; inhibitory buffered, low-Ca*+ saline (1O-v M). -

Pejvakin, a Candidate Stereociliary Rootlet Protein, Regulates Hair Cell Function in a Cell-Autonomous Manner
The Journal of Neuroscience, March 29, 2017 • 37(13):3447–3464 • 3447 Neurobiology of Disease Pejvakin, a Candidate Stereociliary Rootlet Protein, Regulates Hair Cell Function in a Cell-Autonomous Manner X Marcin Kazmierczak,1* Piotr Kazmierczak,2* XAnthony W. Peng,3,4 Suzan L. Harris,1 Prahar Shah,1 Jean-Luc Puel,2 Marc Lenoir,2 XSantos J. Franco,5 and Martin Schwander1 1Department of Cell Biology and Neuroscience, Rutgers the State University of New Jersey, Piscataway, New Jersey 08854, 2Inserm U1051, Institute for Neurosciences of Montpellier, 34091, Montpellier cedex 5, France, 3Department of Otolaryngology, Head and Neck Surgery, Stanford University, Stanford, California 94305, 4Department of Physiology and Biophysics, University of Colorado School of Medicine, Aurora, Colorado 80045, and 5Department of Pediatrics, University of Colorado School of Medicine, Aurora, Colorado 80045 Mutations in the Pejvakin (PJVK) gene are thought to cause auditory neuropathy and hearing loss of cochlear origin by affecting noise-inducedperoxisomeproliferationinauditoryhaircellsandneurons.Herewedemonstratethatlossofpejvakininhaircells,butnot in neurons, causes profound hearing loss and outer hair cell degeneration in mice. Pejvakin binds to and colocalizes with the rootlet component TRIOBP at the base of stereocilia in injectoporated hair cells, a pattern that is disrupted by deafness-associated PJVK mutations. Hair cells of pejvakin-deficient mice develop normal rootlets, but hair bundle morphology and mechanotransduction are affected before the onset of hearing. Some mechanotransducing shorter row stereocilia are missing, whereas the remaining ones exhibit overextended tips and a greater variability in height and width. Unlike previous studies of Pjvk alleles with neuronal dysfunction, our findings reveal a cell-autonomous role of pejvakin in maintaining stereocilia architecture that is critical for hair cell function. -

Molecular Characterization of Hair Cell Metabolism and Tip Link Damage
Molecular characterization of hair cell metabolism and tip link damage by Kateri J. Spinelli A dissertation in partial fulfillment of the requirements for the degree of Doctor of Philosophy Presented to the Neuroscience Graduate Program Oregon Health & Science University School of Medicine February 2012 Table of Contents List of Figures ………………………………………………………………………………………………………..…… iv AcKnowledgments ……………………………………………………………………………………………………… vii Abstract ……………………………………………………………………………………………………………………… ix Chapter 1 – Introduction ............................................................................................. 1 Chapter 2 – Distinct energy metabolism of auditory and vestibular sensory epithelia revealed by quantitative mass spectrometry using MS2 intensities ........................... 13 Abstract ........................................................................................................................ 14 Introduction .................................................................................................................. 15 Results .......................................................................................................................... 17 Label-free quantitation with MS2 intensities ............................................... 17 Quantitation of proteins in chicK auditory and vestibular epithelia ............. 18 Verifying mass spectrometry quantitation ................................................... 21 Comparing protein and mRNA abundance .................................................. -

A Place Principle for Vertigo
Auris Nasus Larynx 35 (2008) 1–10 www.elsevier.com/locate/anl A place principle for vertigo Richard R. Gacek * Department of Otolaryngology, Head and Neck Surgery, University of Massachusetts Medical School, Worcester, MA 01655, USA Received 16 March 2007; accepted 13 April 2007 Available online 24 October 2007 Abstract Objective: To provide a road map of the vestibular labyrinth and its innervation leading to a place principle for different forms of vertigo. Method: The literature describing the anatomy and physiology of the vestibular system was reviewed. Results: Different forms of vertigo may be determined by the type of sense organ, type of ganglion cell and location in the vestibular nerve. Conclusion: Partial lesions (viral) of the vestibular ganglion are manifested as various forms of vertigo. # 2007 Elsevier Ireland Ltd. All rights reserved. Keywords: Vertigo; Vestibular nerve; Pathology Contents 1. Introduction . ............................................................................... 1 2. Sense organ. ............................................................................... 2 3. Ganglion cells ............................................................................... 4 4. Hair cells . ............................................................................... 5 5. Hair cell polarization . ....................................................................... 5 6. Efferent vestibular system ....................................................................... 8 7. A place principle for vertigo . ................................................................. -

The Other Senses
COGS 17 Fall 2009 The Other Senses Mary ET Boyle, Ph.D. Department of Cognitive Science UCSD Peripheral Vestibular Structure: • Inner ear miniaturized accelerometers inertial guidance devices Continually reporting information about: motions and position of head and body Information goes to: brainstem cerebellum somatic sensory cortices 1 Central Vestibular Structure: • Vestibular Nuclei Directly controls motor neurons controlling: extraocular cervical postural Important for: stabilization of gaze head orientation posture during movements The Vestibular Labyrinth: • Main peripheral component Connected with cochlea Uses same specialized hair cells Transduce physical motion into neural impulses head movements inertial effects due to gravity ground-borne vibrations Vestibular endolymph (like cochlear endolymph) high in K+ and low in Na+ 2 Vestibular navigation • Translational movements are in terms of x, y, z Saccule Utricle Rotational movements roll, pitch, yaw • Roll – tumbling left or right -- move your head from your left to your right shoulder • Pitch – nod your head “yes” • Yaw – shake your head “no” Semicircular canals 3 The Vestibular Labyrinth – otolith organs • Two otolith organs - (vestibular sacs) Utricle – hair cells are located on the floor- horizontal plane Saccule – hair cells are located on the wall – vertical motion •Respond to: Information about the position of the head relative to the body utricle cochlea Vestibulocochlear nerve VIII saccule The Vestibular Labyrinth – semicircular canals • Three semicircular canals- (vestibular sacs) Oriented in three planes Ampullae – located at the base of each of the semicircular canals. •Respond to: Rotational accelerations of the head ampullae cochlea Vestibulocochlear nerve VIII 4 The Vestibular Hair cells Similar to auditory hair cells Mechanically gated transduction Channels located at the tips of the stereocilia Otolithic hair cells Scanning EM of calcium carbonate crystals (otoconia) in the utricular macula of the cat. -

Stiffness and Tension Gradients of the Hair Cell's Tip-Link Complex In
RESEARCH ARTICLE Stiffness and tension gradients of the hair cell’s tip-link complex in the mammalian cochlea Me´ lanie Tobin1,2, Atitheb Chaiyasitdhi1,2†, Vincent Michel2,3,4†, Nicolas Michalski2,3,4, Pascal Martin1,2* 1Laboratoire Physico-Chimie Curie, Institut Curie, PSL Research University, CNRS UMR168, Paris, France; 2Sorbonne Universite´, Paris, France; 3Laboratoire de Ge´ne´tique et Physiologie de l’Audition, Institut Pasteur, Paris, France; 4UMRS 1120, Institut National de la Sante´ et de la Recherche Me´dicale (INSERM), Paris, France Abstract Sound analysis by the cochlea relies on frequency tuning of mechanosensory hair cells along a tonotopic axis. To clarify the underlying biophysical mechanism, we have investigated the micromechanical properties of the hair cell’s mechanoreceptive hair bundle within the apical half of the rat cochlea. We studied both inner and outer hair cells, which send nervous signals to the brain and amplify cochlear vibrations, respectively. We find that tonotopy is associated with gradients of stiffness and resting mechanical tension, with steeper gradients for outer hair cells, emphasizing the division of labor between the two hair-cell types. We demonstrate that tension in the tip links that convey force to the mechano-electrical transduction channels increases at reduced Ca2+. Finally, we reveal gradients in stiffness and tension at the level of a single tip link. We conclude that mechanical gradients of the tip-link complex may help specify the characteristic frequency of the hair cell. DOI: https://doi.org/10.7554/eLife.43473.001 *For correspondence: [email protected] †These authors contributed equally to this work Introduction The cochlea—the auditory organ of the inner ear—is endowed with a few thousands of mechanosen- Competing interests: The sory hair cells that are each tuned to detect a characteristic sound frequency (Fettiplace and Kim, authors declare that no 2014). -

Lipid Bilayer Mediates Ion-Channel Cooperativity in a Model of Hair-Cell Mechanotransduction
Lipid bilayer mediates ion-channel cooperativity in a model of hair-cell mechanotransduction Francesco Gianolia, Thomas Rislerb,c,1,2, and Andrei S. Kozlova,1,2 aDepartment of Bioengineering, Imperial College London, London SW7 2AZ, UK; bLaboratoire Physico Chimie Curie, Institut Curie, PSL Research University, CNRS, 26 rue d’Ulm, 75005 Paris, France; cSorbonne Universités, UPMC Univ Paris 06, CNRS, Laboratoire Physico Chimie Curie, 75005 Paris, France This manuscript was compiled on December 25, 2017 Mechanoelectrical transduction in the inner ear is a biophysical pro- constitutes an issue that is often acknowledged (2, 3, 12) but cess underlying the senses of hearing and balance. The key players still unresolved. involved in this process are mechanosensitive ion channels. They The classical model posits a single MET channel connected are located in the stereocilia of hair cells and opened by the tension to the tip link’s upper end, near myosin motors that regulate in specialized molecular springs, the tip links, connecting adjacent tip-link tension (13). Electrophysiological recordings, however, stereocilia. When channels open, the tip links relax, reducing the point to two channels per tip link (14–17), which is in accord hair-bundle stiffness. This gating compliance makes hair cells es- with its dimeric structure (18, 19). Furthermore, high-speed pecially sensitive to small stimuli. The classical explanation for the Ca2+ imaging shows that the channels are located at the tip gating compliance is that the conformational rearrangement of a sin- link’s lower end (17). This result has been corroborated by gle channel directly shortens the tip link. However, to reconcile theo- the expression patterns of key mechanotransduction proteins retical models based on this mechanism with experimental data, an within the hair bundle, which interact with protocadherin-15, unrealistically large structural change of the channel is required. -

The Auditory Periphery 2 – Hair Cell Structure and Transduction
The Auditory Periphery 2 – Hair Cell Structure and Transduction Dr. Elisabeth Glowatzki 955-3877 [email protected] 521 Traylor Building Websites: Promenade ‘round the cochlea (http://www.iurc.montp.inserm.fr/cric/audition/english/start.htm) Auditory Animations, Univ. of Wisconsin (http://www.neurophys.wisc.edu/animations/) Texts (at Welch or Eisenhower): From Sound to Synapse, C. D. Geisler, New York: Oxford Univ. Press, 1998 An Introduction to the Physiology of Hearing, J. O. Pickles, New York: Academic Press, 1982 Fundamentals of Hearing: An Introduction (3rd ed.), W. A. Yost, San Diego: Academic Press, 1994 Hackney CM, Furness DN (1995) Mechanotransduction in vertebrate hair cells: structure and function of the stereociliary bundle. Am. J. Physiol. 268:C1-C13. 1 The Organ of Corti Stephan Blatrix Overview over the organ of Corti One row of inner hair cells (IHCs) and three rows of outer hair cells (OHCs), both with stereocilia bundles. The IHCs are flask-shaped, the OHCs are rod- shaped. Both have stereocilia bundles at the apex and synapses at the base. The OHC stereocilia bundles contact the tectorial membrane, the IHC stereocilia bundles seem not to contact the tectorial membrane. Innervation: 1. IHCs make 95% of afferent glutaminergic synapses (blue). 2. OHCs make 5 % of afferent synapses; their function is unknown (green). 3. OHCs make efferent cholinergic (ACh-activated synapses (red). 4. During development IHCs make have cholinergic synapses (not shown). 2 Cross sections of Organ of Corti of guinea pig. Upper from apex, lower from base of cochlear spiral. R. Pujol. Two histological sections of the organ of Corti, one apical, one basal. -

Mechanical Amplification by Hair Cells in the Semicircular Canals
Mechanical amplification by hair cells in the semicircular canals Richard D. Rabbitta,b,1, Richard Boylec, and Stephen M. Highsteinb aUniversity of Utah, Salt Lake City, UT 84013, bMarine Biological Laboratory, Woods Hole, MA 02543, cBioVIS Center, NASA Ames Research Center, Moffett Field, CA 94035 Edited by Michael V. L. Bennett, Albert Einstein College of Medicine, Bronx, NY, and approved January 11, 2010 (received for review June 16, 2009) Sensory hair cells are the essential mechanotransducers of the inner malian outer hair cells (19). It also has been shown that saccular ear, responsible not only for the transduction of sound and motion hair bundles of the bullfrog (20) and semicircular canal hair stimuli but also, remarkably, for nanomechanical amplification of bundles of the eel (21) move in response to transepithelial electric sensory stimuli. Here we show that semicircular canal hair cells fields and may generate physiologically relevant forces during generate a mechanical nonlinearity in vivo that increases sensitivity transduction. Hair bundles from the saccule exhibit an active to angular motion by amplification at low stimulus strengths. Sensi- process, compressive nonlinearity, that enhances mechanical tivity at high stimulus strengths is linear and shows no evidence of motion for low stimulus strengths (22), and spontaneous oscil- amplification. Results suggest that the mechanical work done by hair lations (23, 24) demonstrating the ability of the hair bundle to do cells contributes ∼97 zJ/cell of amplification per stimulus cycle, improv- mechanical work on the environment. Mechanical power output ing sensitivity to angular velocity stimuli below ∼5°/s (0.3-Hz sinusoidal and amplification by the hair bundle can be evoked by driving the motion).