Prospecting for Cellulolytic Activity in Insect Digestive Fluids
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lepidoptera of North America 5
Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera by Valerio Albu, 1411 E. Sweetbriar Drive Fresno, CA 93720 and Eric Metzler, 1241 Kildale Square North Columbus, OH 43229 April 30, 2004 Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Cover illustration: Blueberry Sphinx (Paonias astylus (Drury)], an eastern endemic. Photo by Valeriu Albu. ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, CO 80523 Abstract A list of 1531 species ofLepidoptera is presented, collected over 15 years (1988 to 2002), in eleven southern West Virginia counties. A variety of collecting methods was used, including netting, light attracting, light trapping and pheromone trapping. The specimens were identified by the currently available pictorial sources and determination keys. Many were also sent to specialists for confirmation or identification. The majority of the data was from Kanawha County, reflecting the area of more intensive sampling effort by the senior author. This imbalance of data between Kanawha County and other counties should even out with further sampling of the area. Key Words: Appalachian Mountains, -

Taxa Names List 6-30-21
Insects and Related Organisms Sorted by Taxa Updated 6/30/21 Order Family Scientific Name Common Name A ACARI Acaridae Acarus siro Linnaeus grain mite ACARI Acaridae Aleuroglyphus ovatus (Troupeau) brownlegged grain mite ACARI Acaridae Rhizoglyphus echinopus (Fumouze & Robin) bulb mite ACARI Acaridae Suidasia nesbitti Hughes scaly grain mite ACARI Acaridae Tyrolichus casei Oudemans cheese mite ACARI Acaridae Tyrophagus putrescentiae (Schrank) mold mite ACARI Analgidae Megninia cubitalis (Mégnin) Feather mite ACARI Argasidae Argas persicus (Oken) Fowl tick ACARI Argasidae Ornithodoros turicata (Dugès) relapsing Fever tick ACARI Argasidae Otobius megnini (Dugès) ear tick ACARI Carpoglyphidae Carpoglyphus lactis (Linnaeus) driedfruit mite ACARI Demodicidae Demodex bovis Stiles cattle Follicle mite ACARI Demodicidae Demodex brevis Bulanova lesser Follicle mite ACARI Demodicidae Demodex canis Leydig dog Follicle mite ACARI Demodicidae Demodex caprae Railliet goat Follicle mite ACARI Demodicidae Demodex cati Mégnin cat Follicle mite ACARI Demodicidae Demodex equi Railliet horse Follicle mite ACARI Demodicidae Demodex folliculorum (Simon) Follicle mite ACARI Demodicidae Demodex ovis Railliet sheep Follicle mite ACARI Demodicidae Demodex phylloides Csokor hog Follicle mite ACARI Dermanyssidae Dermanyssus gallinae (De Geer) chicken mite ACARI Eriophyidae Abacarus hystrix (Nalepa) grain rust mite ACARI Eriophyidae Acalitus essigi (Hassan) redberry mite ACARI Eriophyidae Acalitus gossypii (Banks) cotton blister mite ACARI Eriophyidae Acalitus vaccinii -

Impacts of Native and Non-Native Plants on Urban Insect Communities: Are Native Plants Better Than Non-Natives?
Impacts of Native and Non-native plants on Urban Insect Communities: Are Native Plants Better than Non-natives? by Carl Scott Clem A thesis submitted to the Graduate Faculty of Auburn University in partial fulfillment of the requirements for the Degree of Master of Science Auburn, Alabama December 12, 2015 Key Words: native plants, non-native plants, caterpillars, natural enemies, associational interactions, congeneric plants Copyright 2015 by Carl Scott Clem Approved by David Held, Chair, Associate Professor: Department of Entomology and Plant Pathology Charles Ray, Research Fellow: Department of Entomology and Plant Pathology Debbie Folkerts, Assistant Professor: Department of Biological Sciences Robert Boyd, Professor: Department of Biological Sciences Abstract With continued suburban expansion in the southeastern United States, it is increasingly important to understand urbanization and its impacts on sustainability and natural ecosystems. Expansion of suburbia is often coupled with replacement of native plants by alien ornamental plants such as crepe myrtle, Bradford pear, and Japanese maple. Two projects were conducted for this thesis. The purpose of the first project (Chapter 2) was to conduct an analysis of existing larval Lepidoptera and Symphyta hostplant records in the southeastern United States, comparing their species richness on common native and alien woody plants. We found that, in most cases, native plants support more species of eruciform larvae compared to aliens. Alien congener plant species (those in the same genus as native species) supported more species of larvae than alien, non-congeners. Most of the larvae that feed on alien plants are generalist species. However, most of the specialist species feeding on alien plants use congeners of native plants, providing evidence of a spillover, or false spillover, effect. -

Proceedings of the United States National Museum
PROCEEDINGS OF THE UNITED STATES NATIONAL MUSEUM SMITHSONIAN INSTITUTION U. S. NATIONAL MUSEUM Vol. 93 Washington : 1943 No. 3170 THE NORTH AMERICAN PARASITIC WASPS OF THE GENUS TETRASTICHUS—A CONTRIBUTION TO BIO- LOGICAL CONTROL OF INSECT PESTS By B. D. Burks* The genus Tetrastichus Haliday (Hymenoptera : Eulophidae) in- cludes a large number of species of minute chalcid-flies. These may be either primary parasites or hyperparasites, and they attack a wide variety of hosts (see host list hereinafter), including such destructive pests ib the Hessian fly and the cotton boll weevil and many kinds of fchrips, aphids, midges, leaf miners, scales, tent caterpillars, borers, roaches, beetles, and gall-makers injurious to agriculture, horticulture, and forestry. The}* have been reared from the eggs, larvae, and pupae of other insects, as well as from many plant galls. Economi- cally, therefore, this is an important group of the Chaleidoidea, and a thorough understanding of its species and relationships is desirable. Twenty-three species are herein described for the first time. From a taxonomic standpoint this genus is a difficult one for several reasons. The species are so small that very good microscope equipment is needed for studying them. Specimens are only lightly sclerotized, so that they almost invariably shrivel badly in drying; this tends to conceal or distort their morphological characters. It has not, however, been possible satisfactorily to study specimens pre- served in alcohol or on slides. There is, furthermore, a great lack of good, definite morphological characters for the separation of species Acknowledgment is made to the Illinois State Natural History Survey, Urbana, 111., for granting the author a leave of absence on two occasions, which permitted him to accept a temporary appointment by the U. -
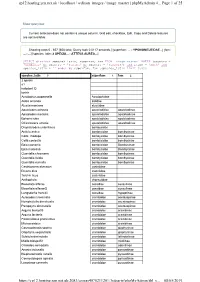
Page 1 of 25 Cp12.Hosting.Zen.Net.Uk / Localhost / Wdixon Images / Image Master | Phpmyadmin 4... 08/05/2019
cp12.hosting.zen.net.uk / localhost / wdixon_images / image_master | phpMyAdmin 4... Page 1 of 25 Show query box Current selection does not contain a unique column. Grid edit, checkbox, Edit, Copy and Delete features are not available. Showing rows 0 - 657 (658 total, Query took 0.0117 seconds.) [superfam: ... - YPONOMEUTIDAE...] [fam: ... - ...] [species_latin: 2 SPECIS... - ATTEVA AUREA...] SELECT distinct species_latin, superfam, fam FROM `image_master` WHERE (country = 'Honduras' or country = 'Panama' or country = 'Pan2018') and itype = 'moth' and species_latin > '' order by superfam, fam ,species_latin limit 0,850 species_latin 3 superfam 1 fam 2 2 specis a1 notodont Q tortrix Acrolophus popeanella Acrolophidae Aidos amanda aididae Alucita montana alucitidae Apatelodes adrastia apatelodidae apatelodinae Apatelodes merlona apatelodidae apatelodinae Ephoria lybia apatelodidae apatelodinae Olceclostera amoria apatelodidae apatelodinae Drepatelodes umbrillinea bombycidae Anticla antica bombycidae bombycinae Colla rhodope bombycidae bombycinae Colla coelestis bombycidae bombycinae Epia casnonia bombycidae Bombycinae Epia muscosa bombycidae Bombycinae Quentalia chromana bombycidae bombycinae Quentalia lividia bombycidae bombycinae Quentalia numalia bombycidae bombycinae Castniomera atymnius castniidae Divana diva castniidae Telchin licus castniidae Anthophyla choreutidae Biocellata alfarae cossidae cossulinae Biocellata alfaraeQ cossidae cossulinae Langsdorfia franckii cossidae hypoptinae Aulacodes traversalis crambidae acentropinae Nymphuliella -

Common Butterflies and Moths (Order Lepidoptera) in the Wichita Mountains and Surrounding Areas
Common Butterflies and Moths (Order Lepidoptera) in the Wichita Mountains and Surrounding Areas Angel Chiri Less than 2% of known species in the U.S. have Entomologist approved common names. Relying on only common names for individual species may lead Introduction to confusion, since more than one common name may exist for the same species, or the With over 11,000 species described in the U.S. same name may be used for more than one and Canada, butterflies and moths are among the species. Using the scientific name, which is the most common and familiar insects. With few same in any language or region, eliminates this exceptions, the adults have two pairs of wings problem. Furthermore, only scientific names are covered with minute and easily dislodgeable used in the scientific literature. Common names scales. The mouthparts consist of a long, are not capitalized. flexible, and coiled proboscis that is used to absorb nectar. Butterflies and skippers are All photos in this guide were taken by the author diurnal, while most moths are nocturnal. using a Canon PowerShot SX110 IS camera. The Lepidoptera undergo a full metamorphosis. Family Pieridae (sulfurs and whites) The larva has a well developed head, with opposable mandibles designed for chewing and Pierids are common, mostly medium-sized, six simple eyes arranged in a semicircle, on each yellowish or white butterflies. The cloudless side of the head. The first three segments (the sulphur, Phoebis sennae has greenish-yellow or thorax) each bears a pair of segmented legs that lemon yellow wings with a spot resembling a end in a single claw. -

BULL0645.Pdf
CONTENTS Introduction Redbud In 'ect Pests Whitc Flannel Moth 2 Redhumped Caterpillar 5 False Unicorn Catcrpillar 7 Fall Wcbworm 9 Redbud Leaffolder 11 Redbud Petiole Gall Midge 13 Redbud Leafhopper 15 Broadno ed Wecvil, 16 Leafcutter Ike' 1 tinging Caterpillar 19 Importance and Control of Redbud Foliage Insects 20 References 20 First Printing 1M, February 2002 Information contained herein is available w all persons regardless of race, color, sex, or national origin. REDBUD INSECTS A GUIDE TO RECOGNITION AND HABITS OF SPECIES DAMAGING FOLIAGE OF ORNAMENTAL REDBUD TREES IN ALABAMA L.L. HYCHE] Introduction REDBUD, OR JUDAS.TREE', is a small, shrub-like, decidu· ous tree that glows commonly and naturally in the (orest under story. It has no value as timber; however, it flowers prolifically and is valued as an omamentallandscape tree. Rowering occurs in late winter/early spring and, for a time, provides welcome color to a landscape still largely bare from winter. Redbud is cultivated and used In many urban and suburban areas of Alabama as an ornamental and/or Jl:reen-space tree. As with many such culti v:ued ornamental plants, it provides favorable habimt for a variN¥ of insects. Rowers provide nectar or pollen to bees, but without harm [0 hlooms or trees. Foliage, however, is prime food for leaf.feeding insects. Damage to leaves pri marily destroys the ornamental quality and envtronmenrol value of the tree; howcvcr, hcavy or complete defoliation can result in loss of growth, dieback of twillS and bmnchcs, or even tree mortality. Dunng research at the Alabama Agricultural Experimem Station dcvotcJ to identification and habiLS of insecLS associated with Alabama trees, 1AssociaTe Pm(c,'ISOr of EnromolOj,ty. -

Forest Vegetation Species Requirements.Pdf
GREAT TRINITY FOREST Forest Vegetation Species Requirements Descriptions of the major forest vegetation types. Volume 15 Table of Contents Section Page # Description of Major Tree Species 1 Ailanthus 2 American basswood 8 American elm 20 Black walnut 30 Black willow 45 Boxelder 53 Bur oak 61 Cedar elm 71 Cedar elm Fact Sheet 78 Chinaberry 80 Weed of the Week: Chinaberry Tree 81 Chinaberry Fact Sheet 82 Chinese tallow tree 84 Weed of the Week: Chinese tallow tree 85 Natural Area Weeds: Chinese tallow (Sapium sebiferum) 86 Chinese Privet 90 Common persimmon 96 Eastern cottonwood 104 Plains cottonwood 113 Eastern redbud 124 Eastern redcedar 131 Green ash 147 Honeylocust 158 Live oak 168 Osage-orange 173 Pecan 184 Post oak 193 Red mulberry 202 Shumard oak 208 Sugarberry 214 Sycamore 221 Texas ash 233 Texas ash Fact Sheet 234 Ash Fact Sheet 237 Texas Buckeye 242 White ash 249 White mulberry 259 Weed of the Week: White mulberry 260 Ohio Perennial and Biennial Weed Guide: White mulberry 261 Mulberry Fact Sheet 264 Winged elm 269 Major Tree Species Literature Cited 275 Understory Species Requirements 277 Aster spp. 278 Roundleaf greenbriar 282 Japanese honeysuckle 285 Poison ivy 289 Western soapberry 292 Field pansy 296 Common blue violet 298 Virginia creeper 301 Wild onion 305 Canada wildrye 309 Virginia wildrye 312 False garlic 316 Understory Plants Literature Cited 319 Description of Major Tree Species Currently there are seven major tree species and a number of minor tree species occupying the Great Trinity Forest. This section will briefly summarize each species and present supporting documentation should there be a deeper interest. -

Checklist of Kansas Insects
CHECKLIST OF KANSAS INSECTS REFERENCE: Insects in Kansas, 3rd edition, 2000 By Stephan White and Glenn Salsbury, Kansas Department of Agriculture Order of Springtails (Collembola) Three-banded Grasshopper – Hadrotettix Family Poduridae trifasciatus Water Springtail – Podura aquatica Toothpick Grasshopper – Leptysma Family Sminthuridae marginicollis Alfalfa Springtail – Sminthurus medialis Differential Grasshopper – Melanoplus differentialis Order of Bristletails and Silverfish Pictured Grasshopper – Dactylotum bicolor (Thysanura) pictum Family Machilidae Family Tridactylidae Machilid – Machilis variabilis Large Sand Cricket – Neotridactylus apicalis Family Lepismatidae Family Tettigoniidae Silverfish – Lepisma saccharina Broad-winged Katydid – Microcentrum rhombifolium Order of Mayflies (Ephemeroptera) Swordbearing Katydid – Neoconocephalus spp. Family Ephemeridae Gladiator Katydid – Orchelimum spp. Giant Mayfly – Hexagenia limbata Family Gryllacrididae Cave Cricket – Ceuthophilis spp. Order of Dragonflies and Damselflies Camel Cricket – Udeopsylla robusta (Odonata) Family Gryllidae Family Aeschnidae Field Cricket – Gryllus spp. Common Green Darner – Anax junius House Cricket – Acheta domesticus Family Libellulidae Snowy Tree Cricket – Oecanthus fultoni Green Clearwing – Erythemis simplicicollis Family Gryllotalpidae Widow – Libellula luctuosa Mole Crickets Ten Spot – Libellula pulchella Amberwing – Perithemis tenera Order of Earwigs (Dermaptera) Whitetail – Plathemis lydia Family Carcinophoridae Family Calopterygidae Earwigs Blackwinged -

Beginner S Guide to Moths of the Midwest Micromoths
0LGZHVW5HJLRQ86$ %HJLQQHU V*XLGHWR0RWKVRIWKH0LGZHVW0LFURPRWKV $QJHOOD0RRUHKRXVH ,OOLQRLV1DWXUH3UHVHUYH&RPPLVVLRQ Photos: Angella Moorehouse ([email protected]). Produced by: Angella Moorehouse with the assistance of Alicia Diaz, Field Museum. Identification assistance provided by: multiple sources (inaturalist.org; bugguide.net) )LHOG0XVHXP &&%<1&/LFHQVHGZRUNVDUHIUHHWRXVHVKDUHUHPL[ZLWKDWWULEXWLRQEXWFRPPHUFLDOXVHRIWKHRULJLQDOZRUN LVQRWSHUPLWWHG >ILHOGJXLGHVILHOGPXVHXPRUJ@>@YHUVLRQ $ERXWWKH%(*,11(5¶6027+62)7+(0,':(67*8,'(6 Most photos were taken in west-central and central Illinois; a few are from eastern Iowa and north-central Wisconsin. Nearly all were posted to identification websites: BugGuide.netDQG iNaturalist.org. Identification help was provided by Aaron Hunt, Steve Nanz, John and Jane Balaban, Chris Grinter, Frank Hitchell, Jason Dombroskie, William H. Taft, Jim Wiker,DQGTerry Harrison as well as others contributing to the websites. Attempts were made to obtain expert verifications for all photos to the field identification level, however, there will be errors. Please contact the author with all corrections Additional assistance was provided by longtime Lepidoptera survey partner, Susan Hargrove. The intention of these guides is to provide the means to compare photographs of living specimens of related moths from the Midwest to aid the citizen scientists with identification in the field for Bio Blitz, Moth-ers Day, and other night lighting events. A taxonomic list to all the species featured is provided at the end along with some field identification tips. :(%6,7(63529,',1*,'(17,),&$7,21,1)250$7,21 BugGuide.net iNaturalist.org Mothphotographersgroup.msstate.edu Insectsofiowa.org centralillinoisinsects.org/weblog/resources/ :+,&+027+*8,'(7286( The moths were split into 6 groups for the purposes of creating smaller guides focusing on similar features of 1 or more superfamilies. -
OSU Extension - Auglaize County Weekly Horticulture Newsletter – 7-10-20
Ohio State University Extension Auglaize County Top of Ohio EERA 208 South Blackhoof Street Wapakoneta, OH 45895-1902 419-739-6580 Phone 419-739-6581 Fax www.auglaize.osu.edu OSU Extension - Auglaize County Weekly Horticulture Newsletter – 7-10-20 Why are the bottoms of my tomatoes black? Tomatoes are beginning to ripen for some. I want to talk about a problem that will likely occur on tomatoes due to the inconsistent rainfall patterns of late. If the bottom or flower end of a tomato has a water-soaked appearance followed by a sunken area turning black, then the fruit has what is called blossom end rot. The lesions may also occur on the sides of the fruit near the flower end of the tomato. In addition to tomato, pepper, eggplant, melon, squash, and cucumber can also get blossom end rot. Blossom end rot is not a disease, it is a physiological disorder caused by an insufficient amount of calcium in the developing fruit. This insufficient amount of calcium can be a function of not enough water. It looks like a disease because the injured area of the fruit is colonized by secondary pathogens. Even though multiple fruits may have blossom end rot, it will NOT spread to other fruits like a typical disease. Is there anything that can be done once you notice the injured fruits? There really is nothing that can be done to solve the problem, unless you catch it very early in the water-soaked lesion stage on the first fruits. At this stage calcium chloride or calcium nitrate can be applied to the foliage. -

Insect Identification Laboratory Annual Report 2017
ID Lab Publication Revised 2018 Insect Identification Laboratory Annual Report 2017 Eric R. Day Theresa A. Dellinger Douglas G. Pfeiffer Department of Entomology, College of Agriculture and Life Sciences Virginia Cooperative Extension Virginia Polytechnic Institute and State University 2018 Virginia Tech 3000-0000 Virginia Cooperative Extension programs and employment are open to all, regardless of age, color, disability, gender, gender identity, gender expression, national origin, political affiliation, race, religion, sexual orientation, genetic information, veteran status, or any other basis protected by law. An equal opportunity/affirmative action employer. Issued in furtherance of Cooperative Extension work, Virginia Polytechnic Institute and State University, Virginia State University, and the U.S. Department of Agriculture cooperating. Edwin J. Jones, Director, Virginia Cooperative Extension, Virginia Tech, Blacksburg; M. Ray McKinnie, Administrator, 1890 Extension Program, Virginia State University, Petersburg. TABLE OF CONTENTS Report Page Monthly Submission Summary 2 Submitter Affiliation Summary 3 Crop Category 4 Host Pest Data 5 County Data 40 INTRODUCTION A total of 1130 requests were received in 2017. This report summarizes the activity of the Insect Identification Laboratory at Virginia Tech for 2017. The laboratory is located in 205A Price Hall and is managed by Eric R. Day, Lab Manager, Theresa Dellinger, Assistant Lab Manager, and Doug Pfeiffer, Extension Entomologist, Department of Entomology, Virginia Tech, Blacksburg, Virginia. Front cover photo: Monocesta coryli by Eric Day Monthly Submission Summary Month # Samples 2017 * January 23 * February 46 * March 84 * April 109 * May 136 * June 164 * July 140 * August 150 * September 100 * October 104 * November 55 * December 19 * Total for 2017 within the specified date range 1,130 * Grand Total 1,130 Submitter Affiliation Summary Submitter Type # of Samples % of Total Alson H.