Technical Data of Cannabinoids
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Extraction of Wax from Sorghum Bran •
i EXTRACTION OF WAX FROM SORGHUM BRAN • 1 HSIEN-WEN HSU <& . ***£/ B. S., National Taiwan University, China, 1951 A THESIS » submitted in partial fulfillment of the requirements for the degree MASTER OF SCIENCE - Department of Chemical Engineering KANSAS STATE COLLEGE OF AGRICULTURE AND APPLIED SCIENCE - 1955 » k Lo i~i ii Q^cu^v^Js TABLE OF CONTENTS INTRODUCTION 1 PREVIOUS WORK 3 MATERIALS 6 GLASS SOXHLET EXTRACTION AND ANALYSIS OF WAX AND OIL IN MISCELLA 7 Procedure of Using Soxhlet Extractor 7 Separation of Wax from Oil by the Acetone Method.. 8 Summary of Results 9 PILOT PLANT EXTRACTION 15 SEPARATION OF WAX AND OIL 16 Effect of Temperature in the Acetone Method 16 Urea Complex Method of Separation. 17 Theory 17 Experimental Procedure 22 Summary of Results 26 COST ESTIMATION OF A WAX RECOVERY PLANT 29 DISCUSSION 30 CONCLUSIONS 35 ACKNOWLEDGMENTS 36 BIBLIOGRAPHY 37 APPENDIX 39 INTRODUCTION i One of the functions of the Agricultural Experiment Station is the never ending search for means of better and fuller utilization of the agri- cultural products that are of particular interest in the state of Kansas. In line with this broad objective, the extraction of economically valuable lipid materials from the sorghum bran has been studied in several theses in the Departments of Chemical Engineering and Chemistry in recent years. Generally the emphasis has b. en placed upon either the chemical and physical properties of these lipides or the equipment design and performance for the solvent ex- traction operation. A brief review of the previous work is presented in the ensuing section. -

Toxicity and Neurotoxic Effects of Monoterpenoids: in Insects and Earthworms Joel R
Entomology Publications Entomology 1-9-1991 Toxicity and Neurotoxic Effects of Monoterpenoids: In Insects and Earthworms Joel R. Coats Iowa State University, [email protected] Laura L. Karr Iowa State University Charles D. Drewes Iowa State University Follow this and additional works at: http://lib.dr.iastate.edu/ent_pubs Part of the Entomology Commons, Environmental Health Commons, Other Animal Sciences Commons, and the Plant Biology Commons The ompc lete bibliographic information for this item can be found at http://lib.dr.iastate.edu/ ent_pubs/377. For information on how to cite this item, please visit http://lib.dr.iastate.edu/ howtocite.html. This Book Chapter is brought to you for free and open access by the Entomology at Iowa State University Digital Repository. It has been accepted for inclusion in Entomology Publications by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Toxicity and Neurotoxic Effects of Monoterpenoids: In Insects and Earthworms Abstract The insecticidal activity of several monoterpenoids from essential oils was evaluated against insect pests. Toxicity tests illustrated the bioactivity of d-limonene, α-terpineol, β-myrcene, linalool, and pulegone against insects, including the house fly, the German cockroach, the rice weevil, and the western corn rootworm. Bioassays were conducted to assess their toxicity via topical application, fumigation, ingestion, and ovicidal exposures. Growth, reproduction and repellency were also evaluated in the German cockroach. Non-invasive electrophysiological recordings were used with an earthworm to investigate neurotoxic effects of the monoterpenoids. Relevant monoterpenoid bioassay results in the literature are also discussed. Disciplines Entomology | Environmental Health | Other Animal Sciences | Plant Biology | Plant Sciences Comments Reprinted (adapted) with permission from Naturally Occurring Pest Bioregulators, 449(20); 305-316. -

The Separation of Three Azeotropes by Extractive Distillation by An-I Yeh A
The separation of three azeotropes by extractive distillation by An-I Yeh A thesis submitted in partial fulfillment of the requirement for the degree of Master of Science in Chemical Engineering Montana State University © Copyright by An-I Yeh (1983) Abstract: Several different kinds of extractive distillation agents were investigated to affect the separation of three binary liquid mixtures, isopropyl ether - acetone, methyl acetate - methanol, and isopropyl ether - methyl ethyl ketone. Because of the small size of the extractive distillation column, relative volatilities were assumed constant and the Fenske equation was used to calculate the relative volatilities and the number of minimum theoretical plates. Dimethyl sulfoxide was found to be a good extractive distillation agent. Extractive distillation when employing a proper agent not only negated the azeotropes of the above mixtures, but also improved the efficiency of separation. This process could reverse the relative volatility of isopropyl ether and acetone. This reversion was also found in the system of methyl acetate and methanol when nitrobenzene was the agent. However, normal distillation curves were obtained for the system of isopropyl ether and methyl ethyl ketone undergoing extractive distillation. In the system of methyl acetate and methanol, the relative volatility decreased as the agents' carbon number increased when glycols were used as the agents. In addition, the oxygen number and the locations of hydroxyl groups in the glycols used were believed to affect the values of relative volatility. An appreciable amount of agent must be maintained in the column to affect separation. When dimethyl sulfoxide was an agent for the three systems studied, the relative volatility increased as the addition rate increased. -

In This Issue
Issue n° 2 MAY 2019 In this issue----- ABACUS project in brief… - ABACUS project in brief The 3-year ABACUS project aims to provide a range of new molecules - Focus on WP1 & WP2 synthetized from microalgae and therefore to bring competitive innovative ingredients based on terpenes for fragrances markets and - Events of interest carotenoids for cosmetics and nutraceutics markets. The concept of ABACUS project associates several interdisciplinary approaches in order to support a high- value product market development stemming from: - Selection and biological engineering of microalgal strains and oriented photosynthesis of terpenoids; - Technological development of algae biomass production systems to optimize cultivation and photosynthesis of terpenoids; - Technological development of the downstream processing steps to reduce time and costs, and to optimize environmental acceptability; - Market development based on new algae-derived ingredients and structuration of new bio-based value chains. To reach its targets, ABACUS takes benefits from a wide range of expertise by gathering 2 large industrial partners (Proteus and Sensient Cosmetics Technologies), 3 algae SMEs (Algafuel, Microphyt and Subitec) and 4 RTOs (CEA, SAMS, CSIC and KIT). Since May 2017, a cooperative work has unfolded between all consortium members whose work is distributed in 10 defined work packages, altogether tailored to reach the objectives by the end of the project. With this second issue of our project’s newsletter, we are pleased to introduce WP1 & WP2 achievements to date. Product and market Market survey acceptances & roadmap Applicability Algae selection Fractionation Process design Up scaling Communication Management Ethics requirements WP1: Solidification of market opportunities and products specifications Two market studies for terpenoid and carotenoid molecules were performed during the first three months of the project. -

European Patent Office of Opposition to That Patent, in Accordance with the Implementing Regulations
(19) TZZ _T (11) EP 2 686 288 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07C 217/56 (2006.01) C07C 217/62 (2006.01) 25.03.2015 Bulletin 2015/13 C07C 227/36 (2006.01) C07C 57/15 (2006.01) C07C 213/08 (2006.01) C07C 219/28 (2006.01) (2006.01) (21) Application number: 11796817.2 C07B 57/00 (22) Date of filing: 11.11.2011 (86) International application number: PCT/IB2011/055039 (87) International publication number: WO 2012/137047 (11.10.2012 Gazette 2012/41) (54) A PROCESS FOR PREPARING FESOTERODINE PROZESS ZUR HERSTELLUNG VON FESOTERODIN PROCÉDÉ DE PRÉPARATION DE FÉSOTÉRODINE (84) Designated Contracting States: • PATIL, Chetan AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Gujarat GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO Vadodara 390003 (IN) PL PT RO RS SE SI SK SM TR • PATEL, Ronak Gujarat (30) Priority: 07.04.2011 IN MM11722011 Vadodara 390003 (IN) • RAVAL, Prashant (43) Date of publication of application: Gujarat 22.01.2014 Bulletin 2014/04 Vadodara 390003 (IN) • PAREKH, Viral (73) Proprietor: Alembic Pharmaceuticals Limited Gujarat Vadodara 390003 (IN) Vadodara 390003 (IN) • SHAH, Hiral (72) Inventors: Gujarat • RAMAN, Jayaraman, Venkat Vadodara 390003 (IN) Gujarat Vadodara 390003 (IN) (74) Representative: Watson, Robert James et al • PATEL, Samir Mewburn Ellis LLP Gujarat 33 Gutter Lane Vadodara 390003 (IN) London EC2V 8AS (GB) • THAKOR, Indrajit Gujarat (56) References cited: Vadodara 390003 (IN) EP-A1- 2 281 801 WO-A2-2009/126844 • LADANI, Mahesh Gujarat Vadodara 390003 (IN) Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. -

Distillation 65 Chem 355 Jasperse DISTILLATION
Distillation 65 Chem 355 Jasperse DISTILLATION Background Distillation is a widely used technique for purifying liquids. The basic distillation process involves heating a liquid such that liquid molecules vaporize. The vapors produced are subsequently passed through a water-cooled condenser. Upon cooling, the vapor returns to it’s liquid phase. The liquid can then be collected. The ability to separate mixtures of liquids depends on differences in volatility (the ability to vaporize). For separation to occur, the vapor that is condensed and collected must be more pure than the original liquid mix. Distillation can be used to remove a volatile solvent from a nonvolatile product; to separate a volatile product from nonvolatile impurities; or to separate two or more volatile products that have sufficiently different boiling points. Vaporization and Boiling When a liquid is placed in a closed container, some of the molecules evaporate into any unoccupied space in the container. Evaporation, which occurs at temperatures below the boiling point of a compound, involves the transition from liquid to vapor of only those molecules at the liquid surface. Evaporation continues until an equilibrium is reached between molecules entering and leaving the liquid and vapor states. The pressure exerted by these gaseous molecules on the walls of the container is the equilibrium vapor pressure. The magnitude of this vapor pressure depends on the physical characteristics of the compound and increases as temperature increases. In an open container, equilibrium is never established, the vapor can simply leave, and the liquid eventually disappears. But whether in an open or closed situation, evaporation occurs only from the surface of the liquid. -
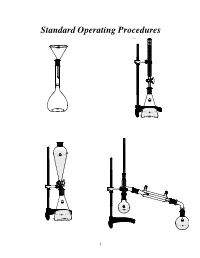
Standard Operating Procedures
Standard Operating Procedures 1 Standard Operating Procedures OVERVIEW In the following laboratory exercises you will be introduced to some of the glassware and tech- niques used by chemists to isolate components from natural or synthetic mixtures and to purify the individual compounds and characterize them by determining some of their physical proper- ties. While working collaboratively with your group members you will become acquainted with: a) Volumetric glassware b) Liquid-liquid extraction apparatus c) Distillation apparatus OBJECTIVES After finishing these sessions and reporting your results to your mentor, you should be able to: • Prepare solutions of exact concentrations • Separate liquid-liquid mixtures • Purify compounds by recrystallization • Separate mixtures by simple and fractional distillation 2 EXPERIMENT 1 Glassware Calibration, Primary and Secondary Standards, and Manual Titrations PART 1. Volumetric Glassware Calibration Volumetric glassware is used to either contain or deliver liquids at a specified temperature. Glassware manufacturers indicate this by inscribing on the volumetric ware the initials TC (to contain) or TD (to deliver) along with the calibration temperature, which is usually 20°C1. Volumetric glassware must be scrupulously clean before use. The presence of streaks or droplets is an indication of the presence of a grease film. To eliminate grease from glassware, scrub with detergent solution, rinse with tap water, and finally rinse with a small portion of distilled water. Volumetric flasks (TC) A volumetric flask has a large round bottom with only one graduation mark positioned on the long narrow neck. Graduation Mark Stopper The position of the mark facilitates the accurate and precise reading of the meniscus. If the flask is used to prepare a solution starting with a solid compound, add small amounts of sol- vent until the entire solid dissolves. -

UCLA Electronic Theses and Dissertations
UCLA UCLA Electronic Theses and Dissertations Title Novel microfluidic technologies for the concentration of radionuclides and radiotracers for positron emission tomography Permalink https://escholarship.org/uc/item/7qb3p6x1 Author Chao, Philip Hong-Sean Publication Date 2019 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA Los Angeles Novel microfluidic technologies for the concentration of radionuclides and radiotracers for positron emission tomography A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Bioengineering by Philip Hong-Sean Chao 2019 © Copyright by Philp Hong-Sean Chao 2019 ABSTRACT OF THE DISSERTATION Novel microfluidic technologies for the concentration of radionuclides and radiotracers for positron emission tomography by Philip Hong-Sean Chao Doctor of Philosophy in Bioengineering University of California, Los Angeles, 2019 Professor Pei-Yu Chiou, Co-Chair Professor Robert Michael van Dam, Co-Chair Positon emission tomography (PET) is an imaging modality capable of visualizing biomolecules in vivo and can be used to aid in disease diagnosis, staging of disease severity, and monitoring of disease response to treatment. PET relies on the use of tracers (i.e. biomolecules labeled with radionuclides) for imaging. Due to the short half-life of the radionuclides used, PET tracer production is typically performed right before an imaging event. Production of a PET tracer can be broken down into three major parts: production of the radionuclide, radiochemical synthesis of the tracer, and, lastly, purification, formulation and quality control testing of the tracer. Several groups, including our own, have looked into leveraging the benefits of microfluidics (reduced system size, finer control of reaction parameters, reduced reagent consumption) towards the production of PET tracers. -

Experiment 2 — Distillation and Gas Chromatography
Chem 21 Fall 2009 Experiment 2 — Distillation and Gas Chromatography _____________________________________________________________________________ Pre-lab preparation (1) Read the supplemental material on distillation theory and techniques from Zubrick, The Organic Chem Lab Survival Manual, and the section on Gas Chromatography from Fessenden, Fessenden, and Feist, Organic Laboratory Techniques, then read this handout carefully. (2) In your notebook, write a short paragraph summarizing what you will be doing in this experiment and what you hope to learn about the efficiencies of the distillation techniques. (3) Sketch the apparatus for the simple and fractional distillations. Your set-up will look much like that shown on p 198 of Zubrick, except that yours will have a simple drip tip in place of the more standard vacuum adaptor. (4) Look up the structures and relevant physical data for the two compounds you will be using. What data are relevant? Read the procedure, think about the data analysis, and decide what you need. (5) Since you have the necessary data, calculate the log of the volatility factor (log α) that you will need for the theoretical plate calculation. Distillation has been used since antiquity to separate the components of mixtures. In one form or another, distillation is used in the manufacture of perfumes, flavorings, liquors, and a variety of other organic chemicals. One of its most important modern applications is in refining crude oil to make fuels, lubricants, and other petrochemicals. The first step in the refining process is separation of crude petroleum into various hydrocarbon fractions by distillation through huge fractionating columns, called distillation towers, that are hundreds of feet high. -

Equation of State for Benzene for Temperatures from the Melting Line up to 725 K with Pressures up to 500 Mpa†
High Temperatures-High Pressures, Vol. 41, pp. 81–97 ©2012 Old City Publishing, Inc. Reprints available directly from the publisher Published by license under the OCP Science imprint, Photocopying permitted by license only a member of the Old City Publishing Group Equation of state for benzene for temperatures from the melting line up to 725 K with pressures up to 500 MPa† MONIKA THOL ,1,2,* ERIC W. Lemm ON 2 AND ROLAND SPAN 1 1Thermodynamics, Ruhr-University Bochum, Universitaetsstrasse 150, 44801 Bochum, Germany 2National Institute of Standards and Technology, 325 Broadway, Boulder, Colorado 80305, USA Received: December 23, 2010. Accepted: April 17, 2011. An equation of state (EOS) is presented for the thermodynamic properties of benzene that is valid from the triple point temperature (278.674 K) to 725 K with pressures up to 500 MPa. The equation is expressed in terms of the Helmholtz energy as a function of temperature and density. This for- mulation can be used for the calculation of all thermodynamic properties. Comparisons to experimental data are given to establish the accuracy of the EOS. The approximate uncertainties (k = 2) of properties calculated with the new equation are 0.1% below T = 350 K and 0.2% above T = 350 K for vapor pressure and liquid density, 1% for saturated vapor density, 0.1% for density up to T = 350 K and p = 100 MPa, 0.1 – 0.5% in density above T = 350 K, 1% for the isobaric and saturated heat capaci- ties, and 0.5% in speed of sound. Deviations in the critical region are higher for all properties except vapor pressure. -

Determination of the Identity of an Unknown Liquid Group # My Name the Date My Period Partner #1 Name Partner #2 Name
Determination of the Identity of an unknown liquid Group # My Name The date My period Partner #1 name Partner #2 name Purpose: The purpose of this lab is to determine the identity of an unknown liquid by measuring its density, melting point, boiling point, and solubility in both water and alcohol, and then comparing the results to the values for known substances. Procedure: 1) Density determination Obtain a 10mL sample of the unknown liquid using a graduated cylinder Determine the mass of the 10mL sample Save the sample for further use 2) Melting point determination Set up an ice bath using a 600mL beaker Obtain a ~5mL sample of the unknown liquid in a clean dry test tube Place a thermometer in the test tube with the sample Place the test tube in the ice water bath Watch for signs of crystallization, noting the temperature of the sample when it occurs Save the sample for further use 3) Boiling point determination Set up a hot water bath using a 250mL beaker Begin heating the water in the beaker Obtain a ~10mL sample of the unknown in a clean, dry test tube Add a boiling stone to the test tube with the unknown Open the computer interface software, using a graph and digit display Place the temperature sensor in the test tube so it is in the unknown liquid Record the temperature of the sample in the test tube using the computer interface Watch for signs of boiling, noting the temperature of the unknown Dispose of the sample in the assigned waste container 4) Solubility determination Obtain two small (~1mL) samples of the unknown in two small test tubes Add an equal amount of deionized into one of the samples Add an equal amount of ethanol into the other Mix both samples thoroughly Compare the samples for solubility Dispose of the samples in the assigned waste container Observations: The unknown is a clear, colorless liquid. -

Terpenoids Commonly Found in Cannabis Sativa Do Not Modulate the Actions of Phytocannabinoids Or Endocannabinoids on TRPA1 and TRPV1 Channels
Cannabis and Cannabinoid Research Volume 5, Number 4, 2020 Mary Ann Liebert, Inc. DOI: 10.1089/can.2019.0099 Terpenoids Commonly Found in Cannabis sativa Do Not Modulate the Actions of Phytocannabinoids or Endocannabinoids on TRPA1 and TRPV1 Channels Marika Heblinski,1,2 Marina Santiago,3 Charlotte Fletcher,1,4 Jordyn Stuart,1,3,4 Mark Connor,3 Iain S. McGregor,1,4 and Jonathon C. Arnold1,2,* Abstract Introduction: Cannabis sativa produces hundreds of bioactive compounds, including cannabinoids and terpe- noids. It has been proposed that cannabinoids act in synergy with terpenoids to produce the entourage effect, a concept used to explain the therapeutic benefits of medicinal cannabis. One molecular explanation for the en- tourage effect is that the terpenoids augment the actions of cannabinoids at their molecular drug targets in cells. We recently reported that terpenoids commonly found in cannabis do not influence the functional effects of D9-tetrahydrocannabinol (D9-THC) on cannabinoid 1 and cannabinoid 2 receptors. The present study aimed to extend on this research by examining whether terpenoids influence the effects of phytocannabinoids and endo- cannabinoids on human transient receptor potential ankyrin 1 (hTRPA1) and human transient receptor potential vanilloid 1 (hTRPV1) channels heterologously expressed in mammalian cells. Materials and Methods: The activity of terpenoids, phytocannabinoids, and endocannabinoids was assessed in inducible HEK Flp-In T-Rex cells transfected with hTRPA1 and hTRPV1 channels, respectively. Real-time changes in intracellular calcium ([Ca]i) were measured using the Calcium 5 dye and a FlexStation 3 plate reader. Results: a-pinene, b-pinene, b-caryophyllene, linalool, limonene, b-myrcene or a-humulene did not affect [Ca]i in hTRPA1 and hTRPV1 overexpressing cells.