A Revision of Rhynchotechum Blume (Gesneriaceae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A New Method of Classification for Tai Textiles
A New Method of Classification for Tai Textiles Patricia Cheesman Textiles, as part of Southeast Asian traditional clothing and material culture, feature as ethnic identification markers in anthropological studies. Textile scholars struggle with the extremely complex variety of textiles of the Tai peoples and presume that each Tai ethnic group has its own unique dress and textile style. This method of classification assumes what Leach calls “an academic fiction … that in a normal ethnographic situation one ordinarily finds distinct tribes distributed about the map in an orderly fashion with clear-cut boundaries between them” (Leach 1964: 290). Instead, we find different ethnic Tai groups living in the same region wearing the same clothing and the same ethnic group in different regions wearing different clothing. For example: the textiles of the Tai Phuan peoples in Vientiane are different to those of the Tai Phuan in Xiang Khoang or Nam Nguem or Sukhothai. At the same time, the Lao and Tai Lue living in the same region in northern Vietnam weave and wear the same textiles. Some may try to explain the phenomena by calling it “stylistic influence”, but the reality is much more profound. The complete repertoire of a people’s style of dress can be exchanged for another and the common element is geography, not ethnicity. The subject of this paper is to bring to light forty years of in-depth research on Tai textiles and clothing in the Lao People’s Democratic Republic (Laos), Thailand and Vietnam to demonstrate that clothing and the historical transformation of practices of social production of textiles are best classified not by ethnicity, but by geographical provenance. -

November 17-2
Tuesday 2 Daily Telegrams November 17, 2020 GOVT. PRIMARY SCHOOL No. TN/DB/PHED/2020/1277 27 SUBHASGRAM - 2 HALDER PARA, SARDAR TIKREY DO OFFICE OF THE EXECUTIVE ENGINEER NSV, SUBHASHGRAM GOVT. PRIMARY SCHOOL PUBLIC HEALTH ENGINEERING DIVISION 28 SUBHASGRAM - 3 DAS PARA, DAKHAIYA PARA DO A.P.W.D., PORT BLAIR NSV, SUBHASHGRAM th SCHOOL TIKREY, SUB CENTER Prothrapur, dated the 13 November 2020. COMMUNITY HALL, 29 KHUDIRAMPUR AREA, STEEL BRIDGE, AAGA DO KHUDIRAMPUR TENDER NOTICE NALLAH, DAM AREA (F) The Executive Engineer, PHED, APWD, Prothrapur invites on behalf of President of India, online Item Rate e- BANGLADESH QUARTER, MEDICAL RAMAKRISHNAG GOVT. PRIMARY SCHOOL tenders (in form of CPWD-8) from the vehicle owners / approved and eligible contractors of APWD and Non APWD 30 COLONY AREA, SAJJAL PARA, R K DO RAM - 1 RAMKRISHNAGRAM Contractors irrespective of their enlistment subject to the condition that they have experience of having successfully GRAM HOUSE SITE completed similar nature of work in terms of cost in any of the government department in A&N Islands and they should GOVT. PRIMARY SCHOOL RAMAKRISHNAG BAIRAGI PARA, MALO PARA, 31 VV PITH, DO not have any adverse remarks for following work RAM - 2 PAHAR KANDA NIT No. Earnest RAMKRISHNAGRAM Sl. Estimated cost Time of Name of work Money RAMAKRISHNAG COMMUNITY HALL, NEAR MAGAR NALLAH WATER TANK No. put to Tender Completion 32 DO Deposit RAM - 3 VKV, RAMKRISHNAGRAM AREA, POLICE TIKREY, DAS PARA VIDYASAGARPAL GOVT. PRIMARY SCHOOL SAITAN TIKRI, PANDEY BAZAAR, 1 NIT NO- R&M of different water pump sets under 33 DO 15/DB/ PHED/ E & M Sub Division attached with EE LI VS PALLY HELIPAD AREA GOVT. -

Sociolinguistic Survey of Mpi in Thailand
Sociolinguistic Survey of Mpi in Thailand Ramzi W. Nahhas SIL International 2007 SIL Electronic Survey Report 2007-016, August 2007 Copyright © 2007 Ramzi W. Nahhas and SIL International All rights reserved 2 Abstract Ramzi W. Nahhas, PhD Survey Unit, Department of Linguistics School of Graduate Studies Payap University/SIL International Chiang Mai, Thailand Mpi is a language spoken mainly in only two villages in Thailand, and possibly in one location in China, as well. Currently, Mpi does not have vernacular literature, and may not have sufficient language vitality to warrant the development of such literature. Since there are only two Mpi villages in Thailand, and they are surrounded by Northern Thai communities, it is reasonable to be concerned about the vitality of the Mpi language. The purposes of this study were to assess the need for vernacular literature development among the Mpi of Northern Thailand and to determine which (if any) Mpi varieties should be developed. This assessment focused on language vitality and bilingualism in Northern Thai. Additionally, lexicostatistics were used to measure lexical similarity between Mpi varieties. Acknowledgments This research was conducted under the auspices of the Payap University Linguistics Department, Chiang Mai, Thailand. The research team consisted of the author, Jenvit Suknaphasawat (SIL International), and Noel Mann (Technical Director, Survey Unit, Payap University Linguistics Department, and SIL International). The fieldwork would not have been possible without the assistance of the residents of Ban Dong (in Phrae Province) and Ban Sakoen (in Nan Province). A number of individuals gave many hours to help the researchers learn about the Mpi people and about their village, and to introduce us to others in their village. -

Dental All Hospital Network 11-03-2021
Dental All Hospital Network 11-03-2021 No Name - Eng Address City Province Openning Hour Tel 51/3 Ngam Wong Wan Road, Khwang Latyao, Chatuchak, 1 Vibhavadi Jatujak Bangkok Everyday (09.00-20.00) 02-941-2800,02-941-2900 Bangkok 10900 2 Bangkok 9 International 362 Rama 2, Khwang Bang Mot, Chomthong, Bangkok 10150 Chomthong Bangkok Everyday (08.00-20.00) 0-2877-1111 1302 Km 3 Bangna-Trat Khwang Bang Na, Bang Na, Bangkok Mon-Fri(09.00-18.00), Sat (09.00- 3 Bangna 1 Bangna Bangkok 02-7468630-9 10260 18.00) 111 Phetkasem 19 Khwang Pak Khlong Phasicharoen, Phasi Mon-Fri (09.00-18.30), Sat-Sun 4 Phyathai 3 Pasicharoen Bangkok 02-467 1111 Charoen, Bangkok 10160 (09.00-16.00) 2677 Pattanakarn Road, Khwang Suan Luang, Suan Luang, 5 Vipharam Suanloung Bangkok Everyday (09.00-20.00) 02-7222500 Ext.2206,2207 Bangkok 10250 2 Soi Soonvijai 7 New Petchaburi Road, Huai Khwang, 6 Bangkok General Hueykwang Bangkok Everyday (08.00-20.00) 02-310-3000 Bangkok 10310 436 Ramkhamhaeng Rd, Khwaeng Hua Mak, Khet Bang Kapi, 7 Ramkhamhaeng Bangkapi Bangkok Everyday ( 08.00- 20.00) 02-7439999 Ext.5493,5494 Krung Thep Maha Nakhon 10240 124 Si Lom, Silom, Khet Bang Rak, Krung Thep Maha 02-625-9000 แจง้ สทิ ธ/ิ์ ท ำนัด * 8 Bangkok Christian Bangrak Bangkok - Nakhon 10500 21250 ทันตกรรม ตอ่ *21220-2 Mon,Tue,Thu (08.00-18.00) 2469/13 New Phetchaburi Khwang Bangkapi, Huai Khwang, 9 Petcharavej huai khwang Bangkok Wed,Fri(08.00-20.00) Sat,Sun 02-718-1515*355 Bangkok 10310 (08.00-16.00) 10 Saint Louis 27 South Sathorn Rd., Yannawa Sathorn Bangkok - 02-838-5555 670/1 Phahonyothin, -

1 Situation Earthquake and Tsunami Date
UNDMT SITUATION REPORT – INDIA EARTHQUAKE AND TSUNAMI DATE: 14.01.05 Highlights of the Situation and Relief /Recovery Efforts The Honorable Prime Minister visited on 8 January 2005 the Tsunami affected areas of A&N Islands and assured of all possible help for rehabilitation and re-construction. An amount of Rs.2 billion was announced as preliminary assistance to be carried out in Andaman and Nicobar Island. The Finance Secretary, Government of India has written to the UNDP Administrator regarding UN assistance for undertaking the work of rebuilding of Infrastructures, both public and private, and for rehabilitation of livelihoods to those affected, as well as enhanced assistance in developing disaster prevention and management systems. The Government has also requested the World Bank and the Asian Development Bank for similar support and assistance. These agencies announced that they would now work with the Government of India to organize a needs assessment, and on that basis develop a program of support for reconstruction and recovery. The affected States/UTs have been sensitized about the apprehension of orphaned children being trafficked. The State Governments/UT Administrations have been requested to keep vigilance about the safety of the children who have been orphaned and women who have been widowed in the Tsunami and prepare list of such orphaned children and widows and monitor their welfare. The Ministry of Social Justice and Empowerment has taken up various measures for relief and rehabilitation of women, children and disabled people affected by Tsunami. Child Help lines have been set up, Short Stay Home for women have been opened; senior officials are coordinating with NGO’s and State Governments for long-term rehabilitation of affected women and children. -

6. Secretary, Ministry of Minority Affairs, 11Trr Floor, Paryavaran Bhawan, CGO Complex, Lodhi Road, New Delhi -110003 7
F.No.14-2/2O2O-[S.L Government of India Ministry of Human Resource Development Department of School Education& Literacy Shastri Bhawan, New Delhi Date;1)lluily,2ozo subject: Minutes ofthe meeting ofthe proiect Approval Board held on 17s March, 2O2O to consider the Annual Work plan & Budget (AWP&B) ZO2O-ZL ol Samagra Shiksha for the UT ofAndaman & Nicobar Islands-reg. The Meeting of Project Approval Board (pAB) of samagra shiksha was held on L7 .03.2020 for under the chairmanship of secretary (sE&L) in New Delhi to consider the Annual work Plan & Budget (Awp&B), zo2o-zt for the ur of Andaman & Nicobar Islands. 2. The undersigned is directed to forward herewith the approved copy of pAB Minutes in respect of samagra shiksha, ur of Andaman & Nicobar Istands for the year 2020-2L for further necessary action. Encl: As above (H. Sonkusare) Under Secretary to the Government of India Phr Ott-25387342 To, 1. Secretary, Ministry of W&CD, 2. Secretary, Ministry of Labour & Employment. 3. Secretary, Ministry of Social fustice & Empowerment. 4. Secretary, Ministry of Tribal Affairs. 5. Secretary, Ministry of Drinking Water & Sanitation, 4th floor, paryavaran Bhawan, CGO compleS Lodhi Road, New Dethi -110O03 6. Secretary, Ministry of Minority Affairs, 11trr floor, paryavaran Bhawan, CGO complex, Lodhi Road, New Delhi -110003 7. secretary, Department of Empowerment of persons with Disabilities, Ministry of Social fustice & Empowerment 8. Deputy Adviser (Education), NITI Aayog 9. Director, NCERT 10. Vice Chancello4 NIEPA Page | 1 11. Chairperson, NCTE, Hans Bhawan, Wing II, 1 Bahadur Shah Zafar Marg, New Delhi -110002 LZ.Yice Chancellor,IGNOU, Maidan Garhi, New Delhi 13. -

The Cultural Politics of Lao Literature, 1941-1975
INVOKING THE PAST: THE CULTURAL POLITICS OF LAO LITERATURE, 1941-1975 A Thesis Presented to the Faculty of the Graduate School of Cornell University In Partial Fulfillment of the Requirements for the Degree of Master of Arts by Chairat Polmuk May 2014 © 2014 Chairat Polmuk ABSTRACT This thesis examines the role of Lao literature in the formation of Lao national identity from 1945 to 1975. In the early 1940s, Lao literary modernity emerged within the specific politico-cultural context of the geopolitical conflict between French Laos and Thailand. As a result, Lao literature and culture became increasingly politicized in colonial cultural policy to counter Thai expansionist nationalism that sought to incorporate Laos into Thai territorial and cultural space. I argue that Lao literature, which was institutionalized by Franco-Lao cultural campaigns between 1941 and 1945, became instrumental to the invention of Lao tradition and served as a way to construct a cultural boundary between Laos and Thailand. Precolonial Lao literature was revitalized as part of Lao national culture; its content and form were also instrumentalized to distinguish Lao identity from that of the Thai. Lao literature was distinguished by the uses of the Lao language, poetic forms, and classical conventions rooted in what was defined as Laos’s own literary culture. In addition, Lao prose fiction, which was made possible in Laos with the rise of print capitalism and an emergent literate social class, offered another mode of “invented tradition.” Despite its presumed novelty in terms of form and content, early Lao prose fiction was highly conventional in its representation of idealized traditional society in opposition to a problematic modern one. -

Urban Axis and City Shape Evaluation Through Spatial Configuration in 'Lan Na' Northern Thailand Historic City
Srinurak and Mishima City Territ Archit (2017) 4:10 DOI 10.1186/s40410-017-0067-z RESEARCH ARTICLE Open Access Urban Axis and City shape evaluation through spatial confguration in ‘Lan Na’ Northern Thailand Historic city Nattasit Srinurak* and Nobuo Mishima Abstract This paper revealed urban axis and city shape identity that infuenced by its concepts and present activities issues in ‘Lan Na’ historic cities in northern Thailand. This study using space syntax technic called axial line analysis combined with GIS analysis, to examines how history geo-politic issues have infuenced to its urban axis and network. Results show urban axis has highly coexisted with its city shape determined through high integrated axial lines. This city shape was, however, defned by its establishment concepts as ‘Sankh’, ‘Traiphum’ and freeform. Diferent shapes of urban axis have been determined by these concepts. As well as the largest public space in some ‘Lan Na’ historic cities, it directly attaches to high integrate lines that represent as urban axis core. However, depending on gradually develop infuences, the function of public space in urban axis core has various types. Mostly, these spaces, have related to Bud- dhism religious usage or colonialism spaces. Settlement pattern using kernel analysis revealed that residents in every city settled in a tranquil area determined by space syntax. Additionally, settlement clusters adjacent to urban axis or high activities trafc. In summary, from reviewed historic cities in ‘Lan Na’, it found that this urban axis could be identi- fed using multi-method. To enhance urban axis in historic cities, both object and subject aspect had to be revealed to apply as delicate historic conservation measures. -

Seed Geometry in the Arecaceae
horticulturae Review Seed Geometry in the Arecaceae Diego Gutiérrez del Pozo 1, José Javier Martín-Gómez 2 , Ángel Tocino 3 and Emilio Cervantes 2,* 1 Departamento de Conservación y Manejo de Vida Silvestre (CYMVIS), Universidad Estatal Amazónica (UEA), Carretera Tena a Puyo Km. 44, Napo EC-150950, Ecuador; [email protected] 2 IRNASA-CSIC, Cordel de Merinas 40, E-37008 Salamanca, Spain; [email protected] 3 Departamento de Matemáticas, Facultad de Ciencias, Universidad de Salamanca, Plaza de la Merced 1–4, 37008 Salamanca, Spain; [email protected] * Correspondence: [email protected]; Tel.: +34-923219606 Received: 31 August 2020; Accepted: 2 October 2020; Published: 7 October 2020 Abstract: Fruit and seed shape are important characteristics in taxonomy providing information on ecological, nutritional, and developmental aspects, but their application requires quantification. We propose a method for seed shape quantification based on the comparison of the bi-dimensional images of the seeds with geometric figures. J index is the percent of similarity of a seed image with a figure taken as a model. Models in shape quantification include geometrical figures (circle, ellipse, oval ::: ) and their derivatives, as well as other figures obtained as geometric representations of algebraic equations. The analysis is based on three sources: Published work, images available on the Internet, and seeds collected or stored in our collections. Some of the models here described are applied for the first time in seed morphology, like the superellipses, a group of bidimensional figures that represent well seed shape in species of the Calamoideae and Phoenix canariensis Hort. ex Chabaud. -

A Brief Nomenclatural Review of Genera and Tribes in Theaceae Linda M
Aliso: A Journal of Systematic and Evolutionary Botany Volume 24 | Issue 1 Article 8 2007 A Brief Nomenclatural Review of Genera and Tribes in Theaceae Linda M. Prince Rancho Santa Ana Botanic Garden, Claremont, California Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Botany Commons, and the Ecology and Evolutionary Biology Commons Recommended Citation Prince, Linda M. (2007) "A Brief Nomenclatural Review of Genera and Tribes in Theaceae," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 24: Iss. 1, Article 8. Available at: http://scholarship.claremont.edu/aliso/vol24/iss1/8 Aliso 24, pp. 105–121 ᭧ 2007, Rancho Santa Ana Botanic Garden A BRIEF NOMENCLATURAL REVIEW OF GENERA AND TRIBES IN THEACEAE LINDA M. PRINCE Rancho Santa Ana Botanic Garden, 1500 North College Ave., Claremont, California 91711-3157, USA ([email protected]) ABSTRACT The angiosperm family Theaceae has been investigated extensively with a rich publication record of anatomical, cytological, paleontological, and palynological data analyses and interpretation. Recent developmental and molecular data sets and the application of cladistic analytical methods support dramatic changes in circumscription at the familial, tribal, and generic levels. Growing interest in the family outside the taxonomic and systematic fields warrants a brief review of the recent nomenclatural history (mainly 20th century), some of the classification systems currently in use, and an explanation of which data support various classification schemes. An abridged bibliography with critical nomen- clatural references is provided. Key words: anatomy, classification, morphology, nomenclature, systematics, Theaceae. INTRODUCTION acters that were restricted to the family and could be used to circumscribe it. -
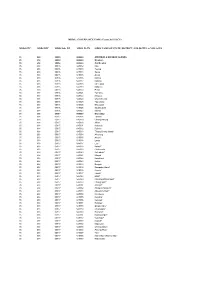
MDDS STC MDDS DTC MDDS Sub DT MDDS PLCN MDDS NAME of STATE, DISTRICT, SUB-DISTTS. & VILLAGES MDDS E-GOVERNANCE CODE (Census
MDDS e-GOVERNANCE CODE (Census 2011 PLCN) MDDS STC MDDS DTC MDDS Sub_DT MDDS PLCN MDDS NAME OF STATE, DISTRICT, SUB-DISTTS. & VILLAGES 35 000 00000 000000 ANDAMAN & NICOBAR ISLANDS 35 638 00000 000000 Nicobars 35 638 05916 000000 Car Nicobar 35 638 05916 645012 Mus 35 638 05916 645013 Teetop 35 638 05916 645014 Sawai 35 638 05916 645015 Arong 35 638 05916 645016 Kimois 35 638 05916 645017 Kakana 35 638 05916 645018 IAF Camp 35 638 05916 645019 Malacca 35 638 05916 645020 Perka 35 638 05916 645021 Tamaloo 35 638 05916 645022 Kinyuka 35 638 05916 645023 Chuckchucha 35 638 05916 645024 Tapoiming 35 638 05916 645025 Big Lapati 35 638 05916 645026 Small Lapati 35 638 05916 645027 Kinmai 35 638 05917 000000 Nancowry 35 638 05917 645028 Tahaila 35 638 05917 645029 Chongkamong 35 638 05917 645030 Alhiat 35 638 05917 645031 Kuitasuk 35 638 05917 645032 Raihion 35 638 05917 645033 Tillang Chong Island* 35 638 05917 645034 Aloorang 35 638 05917 645035 Aloora* 35 638 05917 645036 Enam 35 638 05917 645037 Luxi 35 638 05917 645038 Kalara* 35 638 05917 645039 Chukmachi 35 638 05917 645040 Safedbalu* 35 638 05917 645041 Minyuk 35 638 05917 645042 Kanahinot 35 638 05917 645043 Kalasi 35 638 05917 645044 Bengali 35 638 05917 645045 Bompoka Island* 35 638 05917 645046 Jhoola* 35 638 05917 645047 Jansin* 35 638 05917 645048 Hitlat* 35 638 05917 645049 Mavatapis/Maratapia* 35 638 05917 645050 Chonghipoh* 35 638 05917 645051 Sanaya* 35 638 05917 645052 Alkaipoh/Alkripoh* 35 638 05917 645053 Alhitoth/Alhiloth* 35 638 05917 645054 Katahuwa* 35 638 05917 645055 -

Three New Species of Aspidistra from Vietnam
Wulfenia 23 (2016): 57– 67 Mitteilungen des Kärntner Botanikzentrums Klagenfurt Three new species ofAspidistra (Convallariaceae) from Vietnam Leonid V. Averyanov, H.-J. Tillich, Guo Xiaoming, Quang H. Bui & Khang S. Nguyen Summary: Three new species of the genus Aspidistra, namely A. atrata, A. graminifolia and A. zhangii, discovered recently in Vietnam are described and illustrated as taxa new to science. Data on ecology, phenology, tentative relations, distribution and expected conservation status are recorded for all studied plants. Keywords: Vietnam, Aspidistra, Convallariaceae, new species, flora, plant diversity This paper continues the publication of new results of a successive investigation of species diversity of the genus Aspidistra Ker-Gawl. in Vietnam, which has a significant center of richness in the mainland of southeastern Asia (Lang 1981; Chen & Fang 1982; Wan 1984; Wan & Huang 1987; Li 1988; Fang & Yu 2002; Li & Tang 2002; Bogner & Arnautov 2004; Tillich 2005, 2006, 2014; Brauchler & Ngoc 2005; Tillich et al. 2007; Tillich & Averyanov 2008; Hou et al. 2009; Lin et al. 2009, 2010, 2013a, b, 2014, 2015a, b; Liu et al. 2011; Averyanov & Tillich 2012, 2013, 2014, 2015, 2016; Tillich & Leong-Skornickova 2013; Vislobokov et al. 2013; He et al. 2013; Hu et al. 2014; Leong-Skornickova et al. 2014; Meng et al. 2014; Vislobokov et al. 2014a, b, 2016; Colin 2015; Guo et al. 2015; Vislobokov 2015). Three new species of the genus, Aspidistra atrata, A. graminifolia and A. zhangii discovered recently in Vietnam are described and illustrated as taxa new to science. Data on ecology, phenology, tentative relations, distribution and expected conservation status are recorded for all studied plants.