G:\Haps\Haps Help\Haps Help Ver 2.Wpd
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mechanistic Insights Into the Hydrocyanation Reaction
Mechanistic insights into the hydrocyanation reaction Citation for published version (APA): Bini, L. (2009). Mechanistic insights into the hydrocyanation reaction. Technische Universiteit Eindhoven. https://doi.org/10.6100/IR644067 DOI: 10.6100/IR644067 Document status and date: Published: 01/01/2009 Document Version: Publisher’s PDF, also known as Version of Record (includes final page, issue and volume numbers) Please check the document version of this publication: • A submitted manuscript is the version of the article upon submission and before peer-review. There can be important differences between the submitted version and the official published version of record. People interested in the research are advised to contact the author for the final version of the publication, or visit the DOI to the publisher's website. • The final author version and the galley proof are versions of the publication after peer review. • The final published version features the final layout of the paper including the volume, issue and page numbers. Link to publication General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal. -

4월 24일(목), 09:00~10:30
포스터 I: 4월 24일(목), 09:00~10:30 공업화학: 4월 24일(목), 09:00 ~ 10:30 좌장: 박정훈(동국대) Cleaning system study with mounting activated species of solution using P공업목-1(118) (강원대)권흥수, 이원규 atmospheric pressure plasma 기공구조 조절 제올라이트 촉매를 이용한 액체연료의 흡열량 향상 (고려대)현동훈, 김중연, 김유리, 전병희, P공업목-2(118) (우수 포스터 발표상 후보) 김성현, (국방과학(연))정병훈, 한정식 (고려대)신동건, 김성현, 이창훈, P공업목-3(118) 열 안정성이 우수한 CO 흡착제용 다공성 유무기 복합 지지체 제조 2 조동현, 정현철 (고려대)김유리, 김성현, 전병희, P공업목-4(119) 분산제의 구조에 따른 Jet A-1 연료의 열산화반응 탄소침적물 감소 연구 김중연, 현동훈 (국방과학(연))한정식, 정병훈 Effect of SAA Pretreatment on Temperature of Enzymatic Hydrolysis Using P공업목-5(119) (경기대)장서윤, 박장한, 김준석 Lignocellulosic Biomass Pretreatment and fractionation of Helianthus tuberosus residue by flow-through P공업목-6(119) (경기대)박용철, 박장한, 김준석 reaction (순천대)오성준 (순천대)양지선, 박권필 P공업목-7(119) Carbon Felt 산처리에 의한 바나듐 레독스 흐름전지에 대한 영향 ((주)CNL Energy)나일채, 이정훈 ((주)ETIS)추천호 메틸카바메이트 및 메탄올을 이용한 디메틸카보네이트 제조를 위한 고활성 P공업목-8(120) (한양대)이재홍, 서영웅 촉매 조사 (우수 포스터 발표상 후보) 연속흐름반응기에서 고농도 NaBH 가수분해 반응의 촉매 종류에 따른 P공업목-9(120) 4 (순천대)오성준, 정현승, 박권필 수소발생연구 (순천대)안명원, 곽동영, 복영빈, 박권필 P공업목-10(120) 해조류 종류별 후코산틴 추출 및 염분세척 ((주) ETIS)추천호, 김영숙 (순천대)이세훈, 박권필, ((주)ETIS)김영숙, P공업목-11(120) 효소연료전지에서 Anode 조건에 따른 OCV 변화 추천호, ((주)CNL Energy)나일채 (순천대)정재현, 정회범, 박권필 P공업목-12(121) PEMFC MEA제법에 의한 성능 및 내구성 향상 ((주)CNL Energy)라일채, 이 호 (순천대)정재현, 신은경, 정회범, 박권필 P공업목-13(121) PEM 수전해 과정에서 전극과 전해질 막의 변화 ((주)CNL Energy)라일채, 이 호 (순천대)정재현, 박권필 P공업목-14(121) 직접 개미산 연료전지(DFAFC)의 구동 조건에 따른 성능 비교 ((주)CNL Energy)나일채 (순천대)정재진, 박권필 P공업목-15(121) 가속화도 4.0의 전극, 막, MEA 가속화 기법 개발 (현대자동차)안병기, 김세훈, 고재준 (순천대)정재진, 박권필 P공업목-16(122) 탄화수소막을 사용한 -

Numerical Simulation of a Two-Phase Flow for the Acrylonitrile Electrolytic Adiponitrile Process in a Vertical/Horizontal Electrolysis Cell
energies Article Numerical Simulation of a Two-Phase Flow for the Acrylonitrile Electrolytic Adiponitrile Process in a Vertical/Horizontal Electrolysis Cell Jiin-Yuh Jang * and Yu-Feng Gan Department of Mechanical Engineering, National Cheng-Kung University, Tainan 70101, Taiwan; [email protected] * Correspondence: [email protected]; Tel.: +886-6-2088573 Received: 7 September 2018; Accepted: 6 October 2018; Published: 12 October 2018 Abstract: This paper investigated the effect of oxygen holdup on the current density distribution over the electrode of a vertical/horizontal electrolysis cell with a two-dimensional Eulerian–Eulerian two-phase flow model in the acrylonitrile (AN) electrolytic adiponitrile (ADN) process. The physical models consisted of a vertical/horizontal electrolysis cell 10 mm wide and 600 mm long. The electrical potential difference between the anode and cathode was fixed at 5 V, which corresponded to a uniform current density j = 0.4 A/cm2 without any bubbles released from the electrodes. The effects of different inlet electrolyte velocities (vin = 0.4, 0.6, 1.0 and 1.5 m/s) on the void fraction and the current density distributions were discussed in detail. It is shown that, for a given applied voltage, as the electrolyte velocity is increased, the gas diffusion layer thickness decreased and this resulted in the decrease of the gas void fraction and increase of the corresponding current density; for a given velocity, the current density for a vertical cell was higher than that for a horizontal cell. Furthermore, assuming the release of uniform mass flux for the oxygen results in overestimation of the total gas accumulation mass flow rate by 2.8% and 5.8% and it will also result in underestimation of the current density by 0.3% and 2.4% for a vertical cell and a horizontal cell, respectively. -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

A Quantum Chemical Study Involving Nitrogen Mustards
The Pharmaceutical and Chemical Journal, 2016, 3(4):58-60 Available online www.tpcj.org ISSN: 2349-7092 Research Article CODEN(USA): PCJHBA Formation enthalpy and number of conformers as suitable QSAR descriptors: a quantum chemical study involving nitrogen mustards Robson Fernandes de Farias Universidade Federal do Rio Grande do Norte, Cx. Postal 1664, 59078-970, Natal-RN, Brasil Abstract In the present work, a quantum chemical study (Semi-empirical,PM6 method) is performed using nitrogen mustards (HN1, HN2 and HN3) as subjects in order to demonstrate that there is a close relationship between pharmacological activity and parameters such as formation enthalpy and number of conformers, which could, consequently, be employed as reliable QSAR descriptors. To the studied nitrogen mustards, a very simple equation o o relating log P, ΔH f and the number of conformers (Nc) was found: log P = [(log -ΔH f + logNc)/2]-0.28. Keywords QSAR, Descriptors, Formation enthalpy, Conformers, Semi-empirical, Nitrogen mustards, Log P Introduction It is well known that lipophilicity is a very important molecular descriptor that often correlates well with the bioactivity of chemicals [1]. Hence, lipophilicity, measured as log P, is a key property in quantitative structure activity relationship (QSAR) studies. In this connection, in the pharmaceutical sciences it is a common practice to use log P (the partition coefficient between water and octanol), as a reliable indicator of the hydrophobicity or lipophilicity of (drug) molecules [1-2]. For example, relying primarily on the log P is a sensible strategy in preparing future 18-crown-6 analogs with optimized biological activity [3]. -

The Chemotherapy of Malignant Disease -Practical and Experimental Considerations
Postgrad Med J: first published as 10.1136/pgmj.41.475.268 on 1 May 1965. Downloaded from POSTGRAD. MED. J. (1965), 41,268 THE CHEMOTHERAPY OF MALIGNANT DISEASE -PRACTICAL AND EXPERIMENTAL CONSIDERATIONS JOHN MATTHIAS, M.D., M.R.C.P., F.F.A., R.C.S. Physician, The Royal Marsden Hospital, London, S.W.3. THE TERM chemotherapy was introduced by positively charged alkyl (CH2) radicles of Ehrlich to describe the specific and effective the agent. treatment of infectious disease by chemical (a) The nitrogen mustards: mustine (HN2 substances. It is currently also applied to the 'nitrogen mustard', mechlorethamine, treatment of malignant disease. Unfortunately mustargen), trimustine (Trillekamin no aspect of tumour metabolism has been HN3), chlorambucil (Leukeran, phenyl discovered which has allowed the development butyric mustard), melphalan (Alkeran, of drugs capable of acting specifically upon the phenyl alanine mustard), uramustine malignant cell, so that cytotoxic drugs also (Uracil mustard), cyclophosphamide affect normal cells to a greater or lesser degree. (Endoxan or Cytoxan), mannomustine The most susceptible or sensitive of the normal (DegranoO). tissues are those with the highest rates of cell (b) The ethylenamines: tretamine (trie- turnover and include the haemopoietic and thanomelamine, triethylene melamine, lympho-reticular tissues, the gastro-intestinal TEM), thiotepa (triethylene thiopho- the the testis and the hair epithelium, ovary, sphoramide), triaziquone (Trenimon).by copyright. follicles. (c) The epoxides: triethyleneglycoldigly- Cancer chemotherapy may be said to encom- cidyl ether (Epodyl). pass all treatments of a chemical nature (d) The sulphonic acid esters: busulphan administered to patients with the purpose of (Myleran), mannitol myleran. restricting tumour growth or destroying tumour 2. -

Preparing to Manufacture Hydrogen Peroxide
PREPARING TO MANUFACTURE HYDROGEN PEROXIDE Part of the Hydrogen Peroxide Propulsion Guide The early laboratory preparation of hydrogen peroxide was based on the technique that Thenard used during the initial preparation of hydrogen peroxide. In this technique, barium nitrate, purified by recrystallization, was decomposed by heating in air in a porcelain retort. The resulting oxide was further oxidized by heating in a stream of oxygen to a dull red heat. The barium peroxide which formed was then dampened, ground, and dissolved in hydrochloric acid (nitric acid was used in Thenard’s initial experiments). A slight excess of sulfuric acid was then added to precipitate barium sulfate and regenerate hydrochloric acid. The procedure of barium peroxide solution and sulfate precipitation was repeated several times in the same solution to increase the peroxide concentration (concentrations of up to 33 percent by weight hydrogen peroxide could be achieved in this manner). The concentrated solution containing water, hydrogen peroxide, and hydrochloric acid, along with accumulated impurities, was cooled with ice and saturated with barium peroxide; iron and manganese impurities in the solution were then precipitated out as phosphates. The hydrochloric acid was removed by the addition of silver sulfate and the sulfate ion was removed by the subsequent addition of barium oxide. Further concentration was accomplished by vacuum distillation until “no further density increase occurs.” Thenard reported that 100 w/o hydrogen peroxide (on the basis of density data and the measurement of the volume of oxygen released) could be obtained by this technique. The first record of commercial production of hydrogen peroxide appeared in the 1865 to 1875 period. -

Nerve Agent - Lntellipedia Page 1 Of9 Doc ID : 6637155 (U) Nerve Agent
This document is made available through the declassification efforts and research of John Greenewald, Jr., creator of: The Black Vault The Black Vault is the largest online Freedom of Information Act (FOIA) document clearinghouse in the world. The research efforts here are responsible for the declassification of MILLIONS of pages released by the U.S. Government & Military. Discover the Truth at: http://www.theblackvault.com Nerve Agent - lntellipedia Page 1 of9 Doc ID : 6637155 (U) Nerve Agent UNCLASSIFIED From lntellipedia Nerve Agents (also known as nerve gases, though these chemicals are liquid at room temperature) are a class of phosphorus-containing organic chemicals (organophosphates) that disrupt the mechanism by which nerves transfer messages to organs. The disruption is caused by blocking acetylcholinesterase, an enzyme that normally relaxes the activity of acetylcholine, a neurotransmitter. ...--------- --- -·---- - --- -·-- --- --- Contents • 1 Overview • 2 Biological Effects • 2.1 Mechanism of Action • 2.2 Antidotes • 3 Classes • 3.1 G-Series • 3.2 V-Series • 3.3 Novichok Agents • 3.4 Insecticides • 4 History • 4.1 The Discovery ofNerve Agents • 4.2 The Nazi Mass Production ofTabun • 4.3 Nerve Agents in Nazi Germany • 4.4 The Secret Gets Out • 4.5 Since World War II • 4.6 Ocean Disposal of Chemical Weapons • 5 Popular Culture • 6 References and External Links --------------- ----·-- - Overview As chemical weapons, they are classified as weapons of mass destruction by the United Nations according to UN Resolution 687, and their production and stockpiling was outlawed by the Chemical Weapons Convention of 1993; the Chemical Weapons Convention officially took effect on April 291997. Poisoning by a nerve agent leads to contraction of pupils, profuse salivation, convulsions, involuntary urination and defecation, and eventual death by asphyxiation as control is lost over respiratory muscles. -

Purification and Characterization of a Novel Nitrilase of Rhodococcus
JOURNAL OF BACTERIOLOGY, Sept. 1990, p. 4807-4815 Vol. 172, No. 9 0021-9193/90/094807-09$02.00/0 Copyright X3 1990, American Society for Microbiology Purification and Characterization of a Novel Nitrilase of Rhodococcus rhodochrous K22 That Acts on Aliphatic Nitriles MICHIHIKO KOBAYASHI,* NORIYUKI YANAKA, TORU NAGASAWA, AND HIDEAKI YAMADA Department ofAgricultural Chemistry, Faculty ofAgriculture, Kyoto University, Sakyo-ku, Kyoto 606, Japan Received 18 April 1990/Accepted 8 June 1990 A novel nitrilase that preferentially catalyzes the hydrolysis of aliphatic nitriles to the corresponding carboxylic acids and ammonia was found in the cells of a facultative crotononitrile-utilizing actinomycete isolated from soil. The strain was taxonomically studied and identified as Rhodococcus rhodochrous. The nitrilase was purified, with 9.08% overall recovery, through five steps from a cell extract of the stain. After the last step, the purified enzyme appeared to be homogeneous, as judged by polyacrylamide gel electrophoresis, analytical centrifugation, and double immunodiffusion in agarose. The relative molecular weight values for the native enzyme, estimated from the ultracentrifugal equilibrium and by high-performance liquid chromatog- raphy, were approximately 604,000 + 30,000 and 650,000, respectively, and the enzyme consisted of 15 to 16 subunits identical in molecular weight (41,000). The enzyme acted on aliphatic olefinic nitriles such as crotononitrile and acrylonitrile as the most suitable substrates. The apparent Km values for crotononitrile and acrylonitrile were 18.9 and 1.14 mM, respectively. The nitrilase also catalyzed the direct hydrolysis of saturated aliphatic nitriles, such as valeronitrile, 4-chlorobutyronitrile, and glutaronitrile, to the correspond- ing acids without the formation of amide intermediates. -

Chemical Name Federal P Code CAS Registry Number Acutely
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extremely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extremely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extremely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extremely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extremely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extremely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extremely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extremely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extremely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

Most Common Substances in Chemicals for Different Industries
Table 14 Most common substances by industry Figures for 2009. Substances contained in more than 10 products. A substance can be omitted for secrecy reasons. Industry CAS-no Substance Number of products A01 Agriculture 7732-18-5 Water 458 6484-52-2 Nitric acid ammonium salt 77 57-55-6 1,2-Propanediol 75 64742-65-0 Distillates (petroleum), solvent-dewaxed heavy 74 paraffinic 63148-62-9 Siloxanes and Silicones, di-Me 60 57-13-6 Urea 54 67-63-0 2-Propanol 54 11138-66-2 Xanthan gum 53 7783-20-2 Sulfuric acid diammonium salt 53 7722-76-1 Phosphoric acid, monoammonium salt 52 7487-88-9 Sulfuric acid magnesium salt (1:1) 51 7447-40-7 Potassium chloride 46 56-81-5 1,2,3-Propanetriol 44 7778-80-5 Sulfuric acid dipotassium salt 43 1310-73-2 Sodium hydroxide 41 7647-14-5 Sodium chloride 41 2634-33-5 1,2-Benzisothiazol-3(2H)-one 38 1310-58-3 Potassium hydroxide 33 7757-79-1 Nitric acid potassium salt 33 7785-87-7 Manganese sulfate 32 7664-38-2 Phosphoric acid 32 72623-87-1 Lubricating oils, petroleum, C20-50, 31 hydrotreated neutral oil-based 64741-89-5 Distillates (petroleum), solvent-refined light 29 paraffinic 77-92-9 1,2,3-Propanetricarboxylic acid, 2-hydroxy- 28 8061-51-6 Lignosulfonic acid, sodium salt 28 7778-18-9 Sulfuric acid, calcium salt (1:1) 27 64-17-5 Ethanol 27 14807-96-6 Talc 26 64742-94-5 Solvent naphtha (petroleum), heavy arom. 25 68649-42-3 Phosphorodithioic acid, O,O-di-C1-14-alkyl 24 esters, zinc salts (2:1) 64-02-8 Glycine, N,N'-1,2-ethanediylbis[N- 24 (carboxymethyl)-, tetrasodium salt 64-19-7 Acetic acid 23 99-76-3 Benzoic acid, -
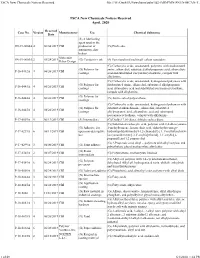
TSCA New Chemicals Notices Received File:///W:/Oneepa/Newchems/Pubs/5D2-IMD/PMN-SNUN-MCAN-T
TSCA New Chemicals Notices Received file:///W:/OneEPA/Newchems/pubs/5d2-IMD/PMN-SNUN-MCAN-T... TSCA New Chemicals Notices Received April, 2020 Received Case No. Version Manufacturer Use Chemical Substance Date (S) A lubricating agent used in the SN-19-0004A 4 06/04/2019 CBI production of (G) Pitch coke automotive disc brakes Molecular SN-19-0005A 2 05/28/2019 (G) Conductive ink (S) Functionalized multiwall carbon nanotubes Rebar Design (G) Carboxylic acids, unsaturated, polymers with disubstituted (G) Polymer for amine, alkanediol, substituted alkylpropanoic acid, alkanedioic P-16-0442A 4 06/26/2019 CBI coatings acid and substituted isocyanatocycloalkane, compds with alkylamine (G) Carboxylic acids, unsaturated, hydrogenated polymers with (G) Polymer for disubstituted amine, alkanediol, substituted alkylpropanoic P-16-0443A 4 06/26/2019 CBI coatings acid, alkanedioic acid and substituted isocyanatocycloalkane, compds with alkylamine (G) Polymer for P-16-0444A 4 06/26/2019 CBI (G) Amine salted polyurethane coatings (G) Carboxylic acids, unsaturated, hydrogenated polymers with (G) Polymer for substituted alkanediamine, alkanediol, substituted P-16-0445A 4 06/26/2019 CBI coatings alkylpropanoic acid, alkanedioic acid and substituted isocyanatocycloalkane, compds with alkylamine P-17-0007A 5 06/13/2019 CBI (S) Intermediate (G) Dialkyl 7,10-dioxa, dithiahexadeca diene (G) Substituted carboxylic acid, polymer with 2,4-diisocyanato- (G) Adhesive for 1-methylbenzene, hexanedioic acid, alpha-hydro-omega- P-17-0239A 6 06/11/2019 CBI open non-descriptive