1 Coastal, Inland Dune and Heath Habitats
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Currents and Waves in the Northern Gulf of Riga
doi:10.5697/oc.54-3.421 Currents and waves OCEANOLOGIA, 54 (3), 2012. in the northern Gulf of pp. 421–447. C Copyright by Riga: measurement and Polish Academy of Sciences, * Institute of Oceanology, long-term hindcast 2012. KEYWORDS Hydrodynamic modelling Water exchange Wave hindcast Wind climate RDCP Baltic Sea Ulo¨ Suursaar⋆ Tiit Kullas Robert Aps Estonian Marine Institute, University of Tartu, M¨aealuse 14, EE–12618 Tallinn, Estonia; e-mail: [email protected] ⋆corresponding author Received 27 February 2012, revised 19 April 2012, accepted 30 April 2012. Abstract Based on measurements of waves and currents obtained for a period of 302 days with a bottom-mounted RDCP (Recording Doppler Current Profiler) at two differently exposed locations, a model for significant wave height was calibrated separately for those locations; in addition, the Gulf of Riga-V¨ainameri 2D model was validated, and the hydrodynamic conditions were studied. Using wind forcing data from the Kihnu meteorological station, a set of current, water exchange and wave hindcasts were obtained for the period 1966–2011. Current patterns in the Gulf and in the straits were wind-dependent with characteristic wind switch directions. The Matsi coast was prone to upwelling in persistent northerly wind conditions. During the * The study was supported by the Estonian target financed project 0104s08, the Estonian Science Foundation grant No 8980 and by the EstKliima project of the European Regional Fund programme No 3.2.0802.11-0043. The complete text of the paper is available at http://www.iopan.gda.pl/oceanologia/ 422 U.¨ Suursaar, T. Kullas, R. -

State of Nature in the Peak District What We Know About the Key Habitats and Species of the Peak District
Nature Peak District State of Nature in the Peak District What we know about the key habitats and species of the Peak District Penny Anderson 2016 On behalf of the Local Nature Partnership Contents 1.1 The background .............................................................................................................................. 4 1.2 The need for a State of Nature Report in the Peak District ............................................................ 6 1.3 Data used ........................................................................................................................................ 6 1.4 The knowledge gaps ....................................................................................................................... 7 1.5 Background to nature in the Peak District....................................................................................... 8 1.6 Habitats in the Peak District .......................................................................................................... 12 1.7 Outline of the report ...................................................................................................................... 12 2 Moorlands .............................................................................................................................................. 14 2.1 Key points ..................................................................................................................................... 14 2.2 Nature and value .......................................................................................................................... -

Latvijas Universitātes Zinātniskie Raksti Acta Universitatis Latviensis
ISSN 1407-2157 Latvijas Universitātes Zinātniskie Raksti Acta Universitatis Latviensis 613 LATVIJAS PURVU VEĢETĀCIJAS KLASIFIKĀCIJA UN DINAMIKA Latvijas Universitāte Latvijas purvu veģetācijas klasifikācija un dinamika Zinātniskie raksti 613. sējums Rīga 1998 -) / Latvijas punu veģetācijas klasifikācija un dinamika: Zinātniskie raksti/Redkolēģija: V.Kreile, M.Laiviņš, A.Namatēva. Rīga: LU, 1998. 92 Ipp. Rakstu krājumā apkopoti pēdējo gadu Latvijas purvu un ezeru krastu veģetācijas pētījumu rezultāti. Analizēti Teicu purva veidošanās apstākļi pēc putekšņu diagrammām. Publicētas purvu augu sabiedrību sintaksonomijas shēmas un sinoptiskās tabulas. Pētījumu rezultātus var izmantot bioloģijas un ģeogrāfijas studenti un citi interesenti. Redakcijas kolēģija: Vija Kreile, Māris Laiviņš, Anita Namatēva © Teicu valsts rezervāts, 1998 PRIEKŠVĀRDS 1997.gada 20.-21.oktobri Teicu rezervātā notika seminārs "Purvu veģetācijas klasifikācija, kartēšana un aizsardzība Latvijā", kurā piedalījās Latvijas Universitātes Bioloģijas un Ģeogrāfijas un Zemes zinātņu fakultāšu, Valsts Ģeoloģijas dienesta, Latvijas Valsts Mežzinātnes institūta "Silava" un Teicu valsts rezervāta speciālisti. Latvijas lielākajā purvu masīvā Teicos notika ekspedīcijas semināra dalībnieku iepazīstināšanai ar sūnu purvu ciņu un lāmu, pārejas un zāļu purvu, ezeru aizaugšanas joslu un palienes pļavu veģetāciju 2 maršrutos: Stiebriņi Kurtavas ezers Šūmāna ezers un Silagals Tolkajas ezers Siksala Islienas ezers. Seminārā tika nolasīti 8 ziņojumi par purvu veģetācijas un floras pētījumiem dažādos Latvijas reģionos, demonstrētas kartes un sintaksonomijas shēmas. Šajā rakstu krājumā publicēti semināra materiāli. Semināra norisi un rakstu krājuma sagatavošanu atbalstīja LR Vides aizsardzības fonds un Teicu valsts rezervāts. SATURS M.Laiviņš. Latvijas ziedaugu un paparžaugu sabiedrību augstākie sintaksoni 7 M.Pakalne. Latvijas purvu veģetācijas raksturojums 23 A. Lācis, L.Kalniņa. Purvu uzbūve un attīstība Teicu valsts rezervātā 39 B.Bambe. Purvu veģetācijas dinamika Teicu rezervātā 56 S.Jermacāne. -

Cycling the Baltic States Saaremaa Virtsu ESTONIA Three Baltic Capitals, Curonian Spit & Saaremaa Island a Kuressaare USSIA R
GUIDED Lithuania – Latvia – Estonia Tallinn Cycling the Baltic States Saaremaa Virtsu ESTONIA Three Baltic capitals, Curonian Spit & Saaremaa Island a Kuressaare USSIA R Baltic Se Jūrmala Sigulda Rīga LATVIA Palanga Hill of Crosses Klaipėda LITHUANIA Ventė Nida Vilnius Kaunas BELARUS RUSSIA Trakai POLAND Tour distances: cycling ~310 km, by coach ~1340 km, by boat ~62 km 11 days / 10 nights TOUR INFORMATION 11 days guided group cycling tour from Vilnius to Tallinn (Code G1) Cycling grade: The Baltic coast and National Parks of Lithuania, Latvia and Estonia explored on highly scenic routes, We rate this trip Easygoing. Daily biking routes mainly on low traffic roads and cycle paths range including the three capital cities – Vilnius, Riga and Tallinn – with their old towns designated by UNESCO from 25 to 60 km (15-37 miles each day). The as the World Heritage Sites; and featuring the previously-closed Curonian Spit and beautiful Estonian terrain is varied and rolling with some gradual island. Travel from Lithuania in the south, through Latvia and on to Estonia in the north, enjoy a great hills on some riding days (some steep ups and variety of towns, villages and landscapes, and get an excellent feel for the different characters of these downs in the Gauja River valley) and dead flat most of the tour. Our walking in the capital towns distinctive countries. is along cobbled streets. Arrival & departure information /Transfers Day 1: Arrive in Vilnius grey herons and cormorants, visit the to Riga. (Cycle ~40 km, bus ~100 km). Airports: Vilnius / Kaunas / Tallinn Welcome meeting at the hotel with Hill of Witches. -

UAL-110 the Estonian Straits
UAL-110 The Estonian Straits Exceptions to the Strait Regime ofInnocent or Transit Passage By Alexander Lott BRILL NIJHOFF LEIDEN I BOSTON UAL-110 Library of Congress Cataloging-in-Publication Data Names: Lott, Alexander, author. Title: The Estonian Straits: Exceptions to the Strait Regime of Innocent or Transit Passage / by Alexander Lott. Description: Leiden; Boston : Koninklijke Brill NV, 2018. I Series: International Straits of the World; Volwne 17 I Based on author's thesis ( doctoral - Tartu Olikool, 2017) issued under title: The Estonian Straits: Exceptions to the Strait Regime of Innocent or Transit Passage. I Includes bibliographical references and index. Identifiers: LCCN 2018001850 (print) I LCCN 201800201s (ebook) I ISBN 9789004365049 (e-book) I ISBN 9789004363861 (hardback: alk. paper) Subjects: LCSH: Straits-Baltic Sea, I Straits--Finland, Gulf of. I Straits--Riga, Gulf of (Latvia and Estonia), I Straits- Estonia, I Straits, I Innocent passage (Law of the sea) I Finland, Gulf of--Intemational status. I Riga, Gulf of (Latvia and Estonia)--Intemational status. Classification: LCC KZ3810 (ehook) I LCC KZ38IO .L68 2018 (print) I DOC 34I.4/48--dc23 LC record available at https://lccn.loc.gov/2018001850 Typeface for the Latin, Greek, and Cyrillic scripts: "Brill". See and download: brill.com/brill-typeface. ISSN 0924-4867 ISBN 978-90-04-36386-l (hardback) ISBN 978-90-04-36504-9 ( e-book) Copyright 2018 by Koninklijke Brill NV, Leiden, The Netherlands. Koninklijke Brill NV incorporates the imprints Brill, Brill Hes & De Graaf, Brill Nijhoff, Brill Rodopi, Brill Sense and Hotei Publishing. All rights reserved. No part of this publication may be reproduced, translated, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without prior written permission from the publisher. -

Managing Molinia? Proceedings of a 3-Day Conference 14-16 September 2015 in Huddersfield, West Yorkshire, UK
Managing Molinia? Proceedings of a 3-day conference 14-16 September 2015 in Huddersfield, West Yorkshire, UK. Edited by Roger Meade National Trust Molinia Conference organising committee at Marsden Moor Estate office. L-R: Alan Stopher, Craig Best, Roger Meade, Nick Pollett and Andrew Underdown. With assistance from Rob Henry, Alyssa Young and Frances DeGiorgio (not in picture). Cover image © Alan Stopher View towards Pule Hill north-eastwards from the route of the old turnpike. Redbrook reservoir is in the middle distance. This is one of the original canal reservoirs which is maintained by Canal & River Trust with the water supplying Yorkshire Water’s customers. A sailing club also uses the amenity. Molinia tussocks dominate the foreground. 2 ‘Managing Molinia’ Conference, 14-16 September 2015, Huddersfield, UK; National Trust, ed. R Meade To cut, or not to cut. A very straightforward question, but so much Foreword more succinct than the answer. This is the dilemma often faced by managers of land for nature conservation where the easiest solution is to just follow what others are doing. As a former habitat specialist for a statutory nature conservation body, I am familiar with the pressures to provide clear guidance and one I remember well is the popular belief that any trees on lowland raised bogs should be cut down and prevented from regrowth. While there is a case for adopting this principle in many situations there are those in which it is not necessary, and is even undesirable from other perspectives such as the trees’ contribution to the landscape. It means that the conservation land manager must not only be aware of the bare bones of the received wisdom, but also of the caveats that make it possible for him or her to arrive at a reasoned judgement for their specific situation. -

Ministero Della Salute Direzione Generale Per L'igiene E La Sicurezza Degli Alimenti E La Nutrizione Ufficio 2 Via Giorgio Ribotta 5- 00144Roma
arsl_ge.alisa.REGISTRO UFFICIALE.I.0012225.25-06-2018 0026792-25/06/2018-DGISAN-MDS-P Trasmissione elettronica N. prot. DGISAN in Docsa/PEC Ministero della Salute Direzione generale per l'igiene e la sicurezza degli alimenti e la nutrizione Ufficio 2 Via Giorgio Ribotta 5- 00144Roma ASSESSORATI ALLA SANITA’ REGIONI E PROVINCIA AUTONOMA DI TRENTO SERVIZI VETERINARI LORO SEDI ASSESSORATO ALL’AGRICOLTURA PROVINCIA AUTONOMA DI BOLZANO SEDE E p.c. ASSICA Pec: [email protected] UNICEB [email protected] [email protected] ASSOCARNI [email protected] FEDERCARNI [email protected] CONSORZIO DEL PROSCIUTTO DI PARMA [email protected]; [email protected] [email protected] CONSORZIO DEL PROSCIUTTO SAN DANIELE [email protected] CARPEGNA PROSCIUTTI S.p.A. [email protected] CONSORZIO DEL PROSCIUTTO DI MODENA [email protected] C.I.A. organizzazione @cia.it CNA [email protected] UNIONALIMENTARI [email protected] A.I.I.P.A. [email protected] CIM –CONSORZIO ITALIANO MACELLATORI Pec: [email protected] DGSAF Ufficio 1 SEDE OGETTO: Aggiornamenti sull’esportazioni di carne fresca suina, prodotti a base di carne suina e prodotti finiti contenti suino dall’ Italia verso la Federazione russa. Si fa seguito alle lettere di questo ufficio n° prot. 15196 del 12 aprile 2018 e N° prot. 10609 del 19 marzo 2018 concernenti l’oggetto per fornire ulteriori aggiornamenti giunti dalla Parte russa con le ultime linee guida Versione del 14/6/2018 e pervenuti per il tramite della Commissione europea, al fine di consentire una esatta compilazione della certificazione veterinaria che deve accompagnare le carni ed i prodotti del settore suino che sono esportati dall’Italia verso la Federazione Russa. -
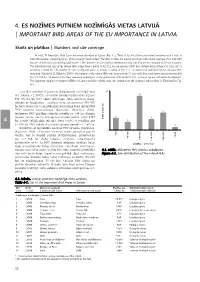
Important Bird Areas of the Eu Importance in Latvia
4. ES NOZĪMES PUTNIEM NOZĪMĪGĀS VIETAS LATVIJĀ | IMPORTANT BIRD AREAS OF THE EU IMPORTANCE IN LATVIA Skaits un platības | Numbers and size coverage In total, 71 Important Bird Areas have been identified in Latvia (Fig. 4-2, Table 4-1), 64 of those are inland territories with a total of 534,056 hectares comprising 8.3% of the country’s land surface. The other 7 areas are marine territories with a total coverage of ca. 339,470 hectares of the Baltic sea and Riga gulf waters. These marine sites are listed for information only, but they are not included in the site accounts. The individual area sizes of the inland IBAs range from 184 ha to 92,322 ha (on average 8345 ha), though most of them (56 sites, 88%) are below 15,000 ha. The number of IBAs in different area size groups is shown in Fig. 4-1. In comparison with the previous national IBA inventory (Raèinskis & Stîpniece 2000), the number of the inland IBAs has increased by 10 sites with their total extent increasing two-fold by 274,368 ha. All marine IBAs have remained unmodified, as the application of the BirdLife IBA criteria at sea are still under development. The long-term changes in number of IBAs in Latvia and their relative area size, compared to the country’s land surface, is illustrated in Fig. 4-3. Latvijâ ir noteikta 71 putniem starptautiski nozîmîgâ vieta (4.1. tabula, 4.2. attçls). 64 no tâm atrodas iekðzemç un aizòem 534 056 ha jeb 8,3% valsts teritorijas. -

Chapter 11 Chapter 11 Wetland Conservation Plan
CHAPTER 11 CHAPTER 11 WETLAND CONSERVATION PLAN 11.1 Wetland Management Conditions 11.1.1 Natural Parks and Reserves (1) Legal conditions for natural parks and reserves 1) National environmental policy The National Environmental Policy Plan (NEPP) for Latvia was accepted by the Cabinet of Ministers of the Republic of Latvia in 1995. NEPP reflects long-term strategy (25~30 years), and has two long-term goals, i) maintenance and protection of existing biodiversity and landscape characteristics of Latvia, and ii) sustainable use of natural resources. 2) National Environmental Action Plan (NEAP) Since Latvia is a country with limited institutional, human, and financial resources, NEAP is incorporated with the National Biodiversity Strategy and the Action Plan, and it will also incorporate implementation of the Ramsar Strategic Plan. NEAP was adopted in 1997, and it emphasizes an establishment of administrative bodies for the Kemeri national park and Lake Engure which include several internationally important wetlands. An elaboration of the Integrated Management Plan for the Lubana Wetland Complex (LWC) is also placed high priority of nature conservation action. 3) Environmental laws and regulations Latvian environmental legal system has been prepared rapidly, and the following laws are relevant to protected areas, especially wetlands. a. The Environmental Protection Law (1991, 1997) determines the general environmental protection objectives, i.e. to ensure preservation of the genetic basis of nature, diversity of biotopes and landscape. It is an umbrella law on nature protection including land use and protection area planning. b. The Law on Specially Protected Nature Areas (1993, 1997) regulates the categories of protected natural areas, the procedure of their establishment and protection. -

2011 Biodiversity Snapshot. Isle of Man Appendices
UK Overseas Territories and Crown Dependencies: 2011 Biodiversity snapshot. Isle of Man: Appendices. Author: Elizabeth Charter Principal Biodiversity Officer (Strategy and Advocacy). Department of Environment, Food and Agriculture, Isle of man. More information available at: www.gov.im/defa/ This section includes a series of appendices that provide additional information relating to that provided in the Isle of Man chapter of the publication: UK Overseas Territories and Crown Dependencies: 2011 Biodiversity snapshot. All information relating to the Isle or Man is available at http://jncc.defra.gov.uk/page-5819 The entire publication is available for download at http://jncc.defra.gov.uk/page-5821 1 Table of Contents Appendix 1: Multilateral Environmental Agreements ..................................................................... 3 Appendix 2 National Wildife Legislation ......................................................................................... 5 Appendix 3: Protected Areas .......................................................................................................... 6 Appendix 4: Institutional Arrangements ........................................................................................ 10 Appendix 5: Research priorities .................................................................................................... 13 Appendix 6 Ecosystem/habitats ................................................................................................... 14 Appendix 7: Species .................................................................................................................... -

1.Jūras Un Iesāļu Augteņu Biotopi
1. Jūras un iesāļu augteņu biotopi Iepriekšējais nosaukums: Piekrastes un halofītiskie biotopi ārējā robeža – tāpēc Latvijā ir saglabājušās daļēji mazskartas (iepriekšējais nosaukums neprecīzi atspoguļoja biotopu un vietām neskartas pludmales, jūras seklūdens un piejūras grupas būtību). platības. Jūras un iesāļu augteņu biotopu grupā ir apvienoti gan jūras biotopi, gan biotopi, kas saistīti ar jūras ietekmi: pludmales Aizsardzības vērtība un citi ar iesāļu jūras ūdeni sezonāli vai neregulāri applūstoši Lai arī šai biotopu grupai Latvijā ir potenciāli ļoti labas izplatības biotopi Piejūras zemienē. Daudzveidīgajā biotopu grupā un attīstības iespējas, tā kopumā ir pieskaitāma ļoti retiem un apvienoti gan īslaicīgi, sezonāli mikrobiotopi, gan relatīvi apdraudētiem biotopiem. Jūras un iesāļu augteņu biotopi ilglaicīgi biotopi, gan dažāda lieluma biotopu kompleksi. nodrošina Baltijas jūras austrumu piekrastei raksturīgo sugu Šie biotopi ir vienoti funkcionējošs komplekss, kas veido un sabiedrību kompleksa saglabāšanos. Šīs sabiedrības veido jūras krastam paralēlas dažāda platuma joslas. Baltijas jūras jūras un vēja pastāvīgai ietekmei, iesāļiem vides apstākļiem piekrastē biotopu joslas ir platākas nekā Rīgas jūras līča un mainīgam mitruma režīmam piemērojušās sugas. Viena krastos. no dažām litorālo augu sugu dabiskajām augtenēm Latvijā. Jūras un tās piekrastes biotopi ir pastāvīgi un vienlaikus ļoti Nelielā sugu skaita un dinamisko apstākļu dēļ šīs sabiedrības dinamiski. Ja pludmali intensīvi pārskalo jūras ūdens un ir ļoti jutīgas pret cilvēka -

Socioeconomic Impact of Mussel Farming in Coastal Areas of Baltic Sea
Socioeconomic Impact of Mussel Farming in Coastal Areas of Baltic Sea Zaiga Ozolina, Ligita Kokaine Kurzeme planning region Published: 2019-04-10 www.balticbluegrowth.eu 1 Socioeconomic Impact of Mussel Farming in Coastal Areas of Baltic Sea About Baltic Blue Growth is a three-year project financed by the European Regional Development Fund. The objective of the project is to remove nutrients from the Baltic Sea by farming and harvesting blue mussels. The farmed mussels will be used for the production of mussel meal, to be used in the feed industry. 18 partners from 7 countries are participating, with representatives from regional and national authorities, research institutions and private companies. The project is coordinated by Region Östergötland (Sweden) and has a total budget of 4,7 M€. Partners - Region Östergötland (SE) - County Administrative Board of Kalmar County (SE) - East regional Aquaculture Centre VCO (SE) - Kalmar municipality (SE) - Kurzeme Planning Region (LV) - Latvian Institute of Aquatic Ecology (LV) - Maritime Institute in Gdańsk (PL) - Ministry of Energy, Agriculture, Environment, Nature and Digitalization of Schleswig- Holstein (DE) - Municipality of Borgholm (DK) - SUBMARINER Network for Blue Growth EEIG (DE) - Swedish University of Agricultural Sciences (SE) - County Administrative Board of Östergötland (SE) - University of Tartu Tartu (EE) - Coastal Research and Management (DE) - Orbicon Ltd. (DK) - Musholm Inc (DK) - Coastal Union Germany EUCC ( DE) - RISE Research institutes of Sweden (SE) This document was produced as an outcome of the Baltic Blue Growth project, WP3, GoA5.4 It was published online at the project’s website www.balticbluegrowth.eu and distributed as an electronic copy to project partners and stakeholders.