The Luminescence of Cdwo4:Tb,Li Crystals Under Synchrotron Excitation at 10 K
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemical List
1 EXHIBIT 1 2 CHEMICAL CLASSIFICATION LIST 3 4 1. Pyrophoric Chemicals 5 1.1. Aluminum alkyls: R3Al, R2AlCl, RAlCl2 6 Examples: Et3Al, Et2AlCl, EtAlCl2, Me3Al, Diethylethoxyaluminium 7 1.2. Grignard Reagents: RMgX (R=alkyl, aryl, vinyl X=halogen) 8 1.3. Lithium Reagents: RLi (R = alkyls, aryls, vinyls) 9 Examples: Butyllithium, Isobutyllithium, sec-Butyllithium, tert-Butyllithium, 10 Ethyllithium, Isopropyllithium, Methyllithium, (Trimethylsilyl)methyllithium, 11 Phenyllithium, 2-Thienyllithium, Vinyllithium, Lithium acetylide ethylenediamine 12 complex, Lithium (trimethylsilyl)acetylide, Lithium phenylacetylide 13 1.4. Zinc Alkyl Reagents: RZnX, R2Zn 14 Examples: Et2Zn 15 1.5. Metal carbonyls: Lithium carbonyl, Nickel tetracarbonyl, Dicobalt octacarbonyl 16 1.6. Metal powders (finely divided): Bismuth, Calcium, Cobalt, Hafnium, Iron, 17 Magnesium, Titanium, Uranium, Zinc, Zirconium 18 1.7. Low Valent Metals: Titanium dichloride 19 1.8. Metal hydrides: Potassium Hydride, Sodium hydride, Lithium Aluminum Hydride, 20 Diethylaluminium hydride, Diisobutylaluminum hydride 21 1.9. Nonmetal hydrides: Arsine, Boranes, Diethylarsine, diethylphosphine, Germane, 22 Phosphine, phenylphosphine, Silane, Methanetellurol (CH3TeH) 23 1.10. Non-metal alkyls: R3B, R3P, R3As; Tributylphosphine, Dichloro(methyl)silane 24 1.11. Used hydrogenation catalysts: Raney nickel, Palladium, Platinum 25 1.12. Activated Copper fuel cell catalysts, e.g. Cu/ZnO/Al2O3 26 1.13. Finely Divided Sulfides: Iron Sulfides (FeS, FeS2, Fe3S4), and Potassium Sulfide 27 (K2S) 28 REFERRAL -

Scintillator Selection
Application Note: Scintillator Selection Table of Contents Page Introduction………………………………………………………… 1,2 Available Scintillators………………………………………………...2 Basic Guide for Scintillator Selection………………………………..3 Figures……………………………………………………………….3,4 Summary……………………………………………………………….4 Introduction Scintillators are a key component for X-Ray Imaging. X-Scan Imaging selects scintillators to optimize for the best resolution, sensitivity, and speed for specific applications. Scintillators are materials that can convert X-ray or gamma ray photons into visible-light photons. The technique of coupling scintillators with photodiodes can be called “scintillation”. Below the scintillator is a CMOS or CCD sensor that is capturing the converted visible photons to create the images output from detectors. In some cases, the scintillator material is thin and sensitive enough to provide high resolution images. As the thickness of the scintillator increases, effects of light scattering and cross talk are factors that can impact the final resolution of a detector. Some Characteristics of scintillators. - Light yield/output: Number of emitted photons per absorbed energy. - Decay time (s): Time required for scintillation emission to decrease to e-1 of its maximum. - Afterglow: Residual light output occurring after the primary decay time of the 1 main luminescent centers. - Stopping power: Attenuation coefficient of the absorbed radiation, for a given thickness of a material. Based on the characteristics of the scintillators above, the ideal scintillators should have high light output, short decay time, minimal afterglow and high stopping power. However, no such perfect scintillator exists. Therefore, we strive to find the ideal scintillator after considering tradeoffs of these combined characteristics for each energy level the users are interested in. X-Scan Imaging utilizes a wide range of scintillator selections based on user’s application needs. -

5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28
Appendix B Classification of common chemicals by chemical band 1 1 EXHIBIT 1 2 CHEMICAL CLASSIFICATION LIST 3 4 1. Pyrophoric Chemicals 5 1.1. Aluminum alkyls: R3A1, R2A1C1, RA1C12 6 Examples: Et3A1, Et2A1C1, EtA.1111C12, Me3A1, Diethylethoxyaluminium 7 1.2. Grignard Reagents: RMgX (R=alkyl, aryl, vinyl X=halogen) 8 1.3. Lithium Reagents: RLi (R 7 alkyls, aryls, vinyls) 9 Examples: Butyllithium, Isobutylthhium, sec-Butyllithium, tert-Butyllithium, 10 Ethyllithium, Isopropyllithium, Methyllithium, (Trimethylsilyl)methyllithium, 11 Phenyllithiurn, 2-Thienyllithium, Vinyllithium, Lithium acetylide ethylenediamine 12 complex, Lithium (trimethylsilyl)acetylide, Lithium phenylacetylide 13 1.4. Zinc Alkyl Reagents: RZnX, R2Zn 14 Examples: Et2Zn 15 1.5. Metal carbonyls: Lithium carbonyl, Nickel tetracarbonyl, Dicobalt octacarbonyl 16 1.6. Metal powders (finely divided): Bismuth, Calcium, Cobalt, Hafnium, Iron, 17 Magnesium, Titanium, Uranium, Zinc, Zirconium 18 1.7. Low Valent Metals: Titanium dichloride 19 1.8. Metal hydrides: Potassium Hydride, Sodium hydride, Lithium Aluminum Hydride, 20 Diethylaluminium hydride, Diisobutylaluminum hydride 21 1.9. Nonmetal hydrides: Arsine, Boranes, Diethylarsine, diethylphosphine, Germane, 22 Phosphine, phenylphosphine, Silane, Methanetellurol (CH3TeH) 23 1.10. Non-metal alkyls: R3B, R3P, R3As; Tributylphosphine, Dichloro(methyl)silane 24 1.11. Used hydrogenation catalysts: Raney nickel, Palladium, Platinum 25 1.12. Activated Copper fuel cell catalysts, e.g. Cu/ZnO/A1203 26 1.13. Finely Divided Sulfides: -

Doping Effects in Nanostructured Cadmium Tungstate Scintillation Films
ARTICLE IN PRESS Journal of Luminescence 121 (2006) 527–534 www.elsevier.com/locate/jlumin Doping effects in nanostructured cadmium tungstate scintillation films Huamei Shanga, Ying Wanga, Brian Milbrathb, Mary Blissb, Guozhong Caoa,Ã aDepartment of Materials Science and Engineering, University of Washington, 302M Roberts Hall, Seattle, WA 98195-2120, USA bPacific Northwest National Laboratory, Richland, WA 99352, USA Received 8 August 2005; received in revised form 28 November 2005; accepted 20 December 2005 Available online 24 January 2006 Abstract This paper reports experimental study on the development of cadmium tungstate scintillator material in the form of nanocrystal films through controlled sol–gel processing and pre-designed doping. We chose cadmium tungstate as a base material for doping and nanostructure development due to its excellent inherent photoluminescence (PL) property, and chemical and thermal stability including non-hydroscopicity. Experimental results revealed that doping with Li+, B3+ and Bi3+ resulted in appreciably reduced grain size and increased density leading to enhanced optical transmittance. Further analyses indicated that PL output changed significantly with the introduction of dopants. The relationships between doping, microstructure, and PL were discussed. r 2006 Elsevier B.V. All rights reserved. PACS: 14.20.Dh Keywords: Cadmium tungstate; Scintillator; Sol–gel processing; Doping; Photoluminescence 1. Introduction widely used in spectrometry and radiometry of radionuclides in extra-low activities, especially Among scintillating materials, cadmium tung- used for computer tomography (CT) [1]. Because 3 state (CdWO4, CWO) is a high-density (7.9 g/cm ), of its capability of radiation capture, CWO has high-Z (atomic number) scintillator with a rela- also been actively studied for applications in X- tively large light yield. -

CPY Document
eADMIUM AN eADMIUM COMPOUNDS Cadmium and cadmium compounds were considered by previous working groups, in 1972,1975 and 1987 (lARC, 1973, 1976, 1987a). New data have since become available, and these are included in the present monograph and have been taken into consideration in the evaluation. The agents considered are metallic cadmium, cadmium alloys and sorne cadmium compounds. 1. Exposure Data 1.1 Chemical and physical data and analysis 1.1.1 Synonyms, trade names and molecular formulae Synonyms, trade names and molecular formulae for cadmium, cadmium-copper alloy and some cadmium compounds are presented in Thble 1. The cadmium compounds shown are those for which data on carcinogenicity or mutagenicity were available or which are commercially important compounds. It is not an exhaustive list and does not necessarily include all of the most commercially important cadmium-containing substances. Table 1. Synonyms (Chemical Abstracts Servce (CAS) names are in italics), trade names and atomic or molecular fonnulae of cadmium and cadmium compounds Chemical name CAS Reg. No. a Synonyms and trade names Formula Cadmium 744-43-9 Cadmium metal; CI 77180 Cd Cadmium acetate 543-90-8 Acetic acid, cadmium salt; bis(acetoxy)- Cd(CH3COO)i (24558-49-4; cadmium; cadmium(II) acetate; cadmium 29398-76-3) diacetate; cadmium ethanoate; CI 77185 Cadmium carbnate 513-78-0 Carbomc acid, cadmium salt; cadmium CdC03 (93820-02-1) carbnate (CdC03); cadmium mono- carbnate; chemcarb; kalcit; mikokalcit; supermikokalcit Cadmium ch/oride 10108-64-2 Cadmium dichloride; -

Hazardous Waste Management Guidebook
Hazardous Waste Management Guidebook FOR UNIVERSTIY AT BUFFALO CAMPUS LABORATORIES Prepared By Environment, Health & Safety Services 220 Winspear Avenue Buffalo, NY 14215 Phone: 716-829-3301 Web: www.ehs.buffalo.edu UB EH&S Hazardous Waste Management Guidebook Table of Contents 1.0 PURPOSE ........................................................................................... 3 2.0 SCOPE ............................................................................................... 4 3.0 DEFINITIONS ...................................................................................... 4 4.0 RESPONSIBILITIES ............................................................................... 5 4.1 EH&S ................................................................................................... 5 4.2 Faculty, Staff, and Students ............................................................. 5 5.0 PROCEDURES ....................................................................................... 6 5.1 Hazardous Waste Determination ................................................... 6 5.1.1 Characteristic Hazardous Wastes .......................................... 7 5.1.2 Listed Hazardous Wastes ........................................................ 9 5.2 Satellite Accumulation of Hazardous Waste ............................ 9 5.2.1 Accumulation Areas ............................................................. 10 5.2.2 Requirements for Hazardous Waste Containers ................ 10 5.2.3 Segregation of Hazardous Wastes ..................................... -

NUCLEAR RADIATION Detector Materials This Symposium, Chaired by E
CERAMICS FROM SOLUTIONS: sol-gel and fine powder technologies During the last thirty years, of submicron dimensions is being having well defined properties at techniques for making ceramic revealed by electron optic methods. significantly lower firing temperatures materials with well defined properties Some applications of sol-gel promises to revolutionize the ceramic have increasingly relied on soluble techniques are well advanced. industry during the coming decades. salts and metal-organic precursors to Extensive work on spherical nuclear As volume increases the cost of most meet exacting chemical purity, fuel forms was presented and the metal organics will decrease homogeneity and microstructural variety of optical coatings available dramatically. When the overall requirements. The resulting was described. A new class of cermets economics are considered, sol-gel and improvements m reactivity, was presented that can be expected to fine particle ceramic technologies in compositional control and process possess unusual electrical properties. combination with advanced heating flexibility have greatly extended our The results of a determination of the methods such as microwave will ability to make novel, utilitarian structure of vanadium pentoxide gels displace many traditional ceramic materials that are well beyond the were presented. Antistatic coatings processing methods. limits believed to exist when only made from these gels have been used naturally occurring minerals were to coat over five million square available. This symposium reviewed meters of photographic film in DON E. HARRISON these developments and it gave an France. Novel switching and display Westinghouse R&D Center indication of the advances that will be devices were also described that are RUSTUM ROY made in the near future. -
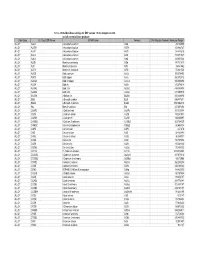
Database Full Listing
16-Nov-06 OLI Data Base Listings for ESP version 7.0.46, Analyzers 2.0.46 and all current alliance products Data Base OLI Tag (ESP) Name IUPAC Name Formula CAS Registry Number Molecular Weight ALLOY AL2U 2-Aluminum uranium Al2U 291.98999 ALLOY AL3TH 3-Aluminum thorium Al3Th 312.982727 ALLOY AL3TI 3-Aluminum titanium Al3Ti 128.824615 ALLOY AL3U 3-Aluminum uranium Al3U 318.971527 ALLOY AL4U 4-Aluminum uranium Al4U 345.953064 ALLOY ALSB Aluminum antimony AlSb 148.731537 ALLOY ALTI Aluminum titanium AlTi 74.861542 ALLOY ALTI3 Aluminum 3-titanium AlTi3 170.621536 ALLOY AUCD Gold cadmium AuCd 309.376495 ALLOY AUCU Gold copper AuCu 260.512512 ALLOY AUCU3 Gold 3-copper AuCu3 387.604492 ALLOY AUSN Gold tin AuSn 315.676514 ALLOY AUSN2 Gold 2-tin AuSn2 434.386505 ALLOY AUSN4 Gold 4-tin AuSn4 671.806519 ALLOY BA2SN 2-Barium tin Ba2Sn 393.369995 ALLOY BI2U 2-Bismuth uranium Bi2U 655.987671 ALLOY BI4U3 4-Bismuth 3-uranium Bi4U3 1550.002319 ALLOY BIU Bismuth uranium BiU 447.007294 ALLOY CA2PB 2-Calcium lead Ca2Pb 287.355988 ALLOY CA2SI 2-Calcium silicon Ca2Si 108.241501 ALLOY CA2SN 2-Calcium tin Ca2Sn 198.865997 ALLOY CA3SB2 3-Calcium 2-antimony Ca3Sb2 363.734009 ALLOY CAMG2 Calcium 2-magnesium CaMg2 88.688004 ALLOY CAPB Calcium lead CaPb 247.278 ALLOY CASI Calcium silicon CaSi 68.163498 ALLOY CASI2 Calcium 2-silicon CaSi2 96.249001 ALLOY CASN Calcium tin CaSn 158.787994 ALLOY CAZN Calcium zinc CaZn 105.468002 ALLOY CAZN2 Calcium 2-zinc CaZn2 170.858002 ALLOY CD11U 11-Cadmium uranium Cd11U 1474.536865 ALLOY CD3AS2 3-Cadmium 2-arsenic As2Cd3 487.073212 -
Standard X-Ray Diffraction Powder Patterns
E^l Admin. NBS MONOGRAPH 25—SECTION 5 Refecii^M not to be ^ferlrom the library. Standard X-ray Diffraction Powder Patterns ^\ / U.S. DEPARTMENT OF COMMERCE S NATIONAL BUREAU OF STANDARDS THE NATIONAL BUREAU OF STANDARDS The National Bureau of Standards^ provides measurement and technical information services essential to the efficiency and effectiveness of the work of the Nation's scientists and engineers. The Bureau serves also as a focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. To accomplish this mission, the Bureau is organized into three institutes covering broad program areas of research and services: THE INSTITUTE FOR BASIC STANDARDS . provides the central basis within the United States for a complete and consistent system of physical measurements, coordinates that system with the measurement systems of other nations, and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation's scientific community, industry, and commerce. This Institute comprises a series of divisions, each serving a classical subject matter area: —Applied Mathematics—Electricity—Metrology—Mechanics—Heat—Atomic Physics—Physical Chemistry—Radiation Physics— -Laboratory Astrophysics^—Radio Standards Laboratory,^ which includes Radio Standards Physics and Radio Standards Engineering—Office of Standard Refer- ence Data. THE INSTITUTE FOR MATERIALS RESEARCH . conducts materials research and provides associated materials services including mainly reference materials and data on the properties of ma- terials. Beyond its direct interest to the Nation's scientists and engineers, this Institute yields services which are essential to the advancement of technology in industry and commerce. -
The Solubility of Some Sparingly Soluble Salts of Zinc and Cadmium in Water and in Aqueous Electrolyte Solutions
The Solubility of Some Sparingly Soluble Salts of Zinc and Cadmium in Water and in Aqueous Electrolyte Solutions Cite as: Journal of Physical and Chemical Reference Data 21, 941 (1992); https://doi.org/10.1063/1.555909 Submitted: 12 February 1991 . Published Online: 15 October 2009 H. Lawrence Clever, M. Elizabeth Derrick, and Susan A. Johnson ARTICLES YOU MAY BE INTERESTED IN The solubility of some sparingly soluble lead salts: An evaluation of the solubility in water and aqueous electrolyte solution Journal of Physical and Chemical Reference Data 9, 751 (1980); https:// doi.org/10.1063/1.555628 The Solubility of Mercury and Some Sparingly Soluble Mercury Salts in Water and Aqueous Electrolyte Solutions Journal of Physical and Chemical Reference Data 14, 631 (1985); https:// doi.org/10.1063/1.555732 IUPAC-NIST Solubility Data Series. 92. Metal Carbonates. Part 1. Solubility and Related Thermodynamic Quantities of Cadmium(II) Carbonate in Aqueous Systems Journal of Physical and Chemical Reference Data 40, 043104 (2011); https:// doi.org/10.1063/1.3645087 Journal of Physical and Chemical Reference Data 21, 941 (1992); https://doi.org/10.1063/1.555909 21, 941 © 1992 American Institute of Physics for the National Institute of Standards and Technology. The Solubility of Some Sparingly Soluble Salts of Zinc and Cadmium in Water and in Aqueous Electrolyte Solutions H. Lawrence Clever, M. Elizabeth Derrick-, and Susan A. Johnson Solubility Research and In/ormation Project, Department 0/ Chemistry, Emory University, Atlanta, GA 30322 Received February 12, 1991; revised manuscript received March 4, 1992 The literature on the solubility of the sparingly soluble inorganic salts of zinc and cadmium in water and in aqueous electrolyte solutions has been reviewed. -

Exposure Data 1.1 Chemical and Physical Data and Analysis
eADMIUM AN eADMIUM COMPOUNDS Cadmium and cadmium compounds were considered by previous working groups, in 1972,1975 and 1987 (lARC, 1973, 1976, 1987a). New data have since become available, and these are included in the present monograph and have been taken into consideration in the evaluation. The agents considered are metallic cadmium, cadmium alloys and sorne cadmium compounds. 1. Exposure Data 1.1 Chemical and physical data and analysis 1.1.1 Synonyms, trade names and molecular formulae Synonyms, trade names and molecular formulae for cadmium, cadmium-copper alloy and some cadmium compounds are presented in Thble 1. The cadmium compounds shown are those for which data on carcinogenicity or mutagenicity were available or which are commercially important compounds. It is not an exhaustive list and does not necessarily include all of the most commercially important cadmium-containing substances. Table 1. Synonyms (Chemical Abstracts Servce (CAS) names are in italics), trade names and atomic or molecular fonnulae of cadmium and cadmium compounds Chemical name CAS Reg. No. a Synonyms and trade names Formula Cadmium 744-43-9 Cadmium metal; CI 77180 Cd Cadmium acetate 543-90-8 Acetic acid, cadmium salt; bis(acetoxy)- Cd(CH3COO)i (24558-49-4; cadmium; cadmium(II) acetate; cadmium 29398-76-3) diacetate; cadmium ethanoate; CI 77185 Cadmium carbnate 513-78-0 Carbomc acid, cadmium salt; cadmium CdC03 (93820-02-1) carbnate (CdC03); cadmium mono- carbnate; chemcarb; kalcit; mikokalcit; supermikokalcit Cadmium ch/oride 10108-64-2 Cadmium dichloride; -

A Study of the Deterioration of X-Ray Fluoroscopic Screens
AN ABSTRACT OF THE THESIS OF WILLIAM STUART PROPERZIO for the MASTER OF SCIENCE (Name) (Degree) in RADIOLOGICAL PHYSICS presented on August 3, 1967 (Major) (Date) Title: A STUDY OF THE DETERIORATION OF X -RAY FLUORO- SCOPIC SCREENS Abstract approved: Dr. E. Dale Trout From the time when fluoroscopic screens were first used there have been scattered references to reduction in their light output due to age, use, etc. References are scarce and usually devoid of quantitative data. A long -range program has been set up in an effort to arrive at some meaningful information. A group of 24 fluoroscopic screens were used in this study, 12 each from two manufacturers. All screens were of the zinc- cadmium sulfide phosphor type mounted on plastic backing material. The r screens were divided into test groups and subjected to exposure of . X -ray, sunlight, incandescent lamps and fluorescent lamps. Test conditions were so designed that they could be related to some real- istic exposure to which the screen in a diagnostic unit might be sub- jected. To determine their relative condition, measurements of the light output of all the screens were taken on a monthly basis. These measurements were made under controlled conditions utilizing a specially- constructed special X -ray unit and a direct - light meter employing a photomultiplier tube as the detector. By use of a direct - reading instrument calibrated in foot -lamberts, measure- ments were made independently of the sensitivity of the observer's eye and required no time -consuming matching of brightness. Cali- bration of the light meter was maintained by use of a regulated brightness source.