Brucella Suis Bacteremia Misidentified As Ochrobactrum Anthropi by the VITEK 2 System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Antibiotic Resistant Bacteria in Water Environments in Louisville, Kentucky
University of Louisville ThinkIR: The University of Louisville's Institutional Repository College of Arts & Sciences Senior Honors Theses College of Arts & Sciences 5-2018 Antibiotic resistant bacteria in water environments in Louisville, Kentucky. Amy Priest University of Louisville Follow this and additional works at: https://ir.library.louisville.edu/honors Part of the Environmental Microbiology and Microbial Ecology Commons Recommended Citation Priest, Amy, "Antibiotic resistant bacteria in water environments in Louisville, Kentucky." (2018). College of Arts & Sciences Senior Honors Theses. Paper 173. Retrieved from https://ir.library.louisville.edu/honors/173 This Senior Honors Thesis is brought to you for free and open access by the College of Arts & Sciences at ThinkIR: The University of Louisville's Institutional Repository. It has been accepted for inclusion in College of Arts & Sciences Senior Honors Theses by an authorized administrator of ThinkIR: The University of Louisville's Institutional Repository. This title appears here courtesy of the author, who has retained all other copyrights. For more information, please contact [email protected]. Antibiotic Resistant Bacteria in Water Environments in Louisville, Kentucky: An Analysis of Common Genera and Community Diversity By Amy Priest Submitted in partial fulfillment of the requirements for Graduation summa cum laude and for Graduation with Honors from the Department of Biology University of Louisville May, 2018 1 Table of Contents Abstract .......................................................................................................................................... -

Genital Brucella Suis Biovar 2 Infection of Wild Boar (Sus Scrofa) Hunted in Tuscany (Italy)
microorganisms Article Genital Brucella suis Biovar 2 Infection of Wild Boar (Sus scrofa) Hunted in Tuscany (Italy) Giovanni Cilia * , Filippo Fratini , Barbara Turchi, Marta Angelini, Domenico Cerri and Fabrizio Bertelloni Department of Veterinary Science, University of Pisa, Viale delle Piagge 2, 56124 Pisa, Italy; fi[email protected] (F.F.); [email protected] (B.T.); [email protected] (M.A.); [email protected] (D.C.); [email protected] (F.B.) * Correspondence: [email protected] Abstract: Brucellosis is a zoonosis caused by different Brucella species. Wild boar (Sus scrofa) could be infected by some species and represents an important reservoir, especially for B. suis biovar 2. This study aimed to investigate the prevalence of Brucella spp. by serological and molecular assays in wild boar hunted in Tuscany (Italy) during two hunting seasons. From 287 animals, sera, lymph nodes, livers, spleens, and reproductive system organs were collected. Within sera, 16 (5.74%) were positive to both rose bengal test (RBT) and complement fixation test (CFT), with titres ranging from 1:4 to 1:16 (corresponding to 20 and 80 ICFTU/mL, respectively). Brucella spp. DNA was detected in four lymph nodes (1.40%), five epididymides (1.74%), and one fetus pool (2.22%). All positive PCR samples belonged to Brucella suis biovar 2. The results of this investigation confirmed that wild boar represents a host for B. suis biovar. 2 and plays an important role in the epidemiology of brucellosis in central Italy. Additionally, epididymis localization confirms the possible venereal transmission. Citation: Cilia, G.; Fratini, F.; Turchi, B.; Angelini, M.; Cerri, D.; Bertelloni, Keywords: Brucella suis biovar 2; wild boar; surveillance; epidemiology; reproductive system F. -

Environment-Dependent Variation in Gut Microbiota of an Oviparous Lizard (Calotes Versicolor)
animals Article Environment-Dependent Variation in Gut Microbiota of an Oviparous Lizard (Calotes versicolor) Lin Zhang 1,2,*,† , Fang Yang 3,†, Ning Li 4 and Buddhi Dayananda 5 1 School of Basic Medical Sciences, Hubei University of Chinese Medicine, Wuhan 430065, China 2 State Key Laboratory of Microbial Technology, Institute of Microbial Technology, Shandong University, Qingdao 266237, China 3 School of Laboratory Medicine, Hubei University of Chinese Medicine, Wuhan 430065, China; [email protected] 4 College of Food Science, Nanjing Xiaozhuang University, Nanjing 211171, China; [email protected] 5 School of Agriculture and Food Sciences, The University of Queensland, Brisbane, QLD 4072, Australia; [email protected] * Correspondence: [email protected] † These authors contributed equally to this work. Simple Summary: The different gut sections potentially provide different habitats for gut microbiota. We found that Bacteroidetes, Firmicutes, and Proteobacteria were the three primary phyla in gut micro- biota of C. versicolor. The relative abundance of dominant phyla Bacteroidetes and Firmicutes exhibited an increasing trend from the small intestine to the large intestine, and there was a higher abun- dance of genus Bacteroides (Class: Bacteroidia), Coprobacillus and Eubacterium (Class: Erysipelotrichia), Parabacteroides (Family: Porphyromonadaceae) and Ruminococcus (Family: Lachnospiraceae), and Family Odoribacteraceae and Rikenellaceae in the hindgut, and some metabolic pathways were higher in the hindgut. Our results reveal the variations of gut microbiota composition and metabolic pathways in Citation: Zhang, L.; Yang, F.; Li, N.; different parts of the lizards’ intestine. Dayananda, B. Environment- Dependent Variation in Gut Abstract: Vertebrates maintain complex symbiotic relationships with microbiota living within their Microbiota of an Oviparous Lizard gastrointestinal tracts which reflects the ecological and evolutionary relationship between hosts and (Calotes versicolor). -

Characterization of Environmental and Cultivable Antibiotic- Resistant Microbial Communities Associated with Wastewater Treatment
antibiotics Article Characterization of Environmental and Cultivable Antibiotic- Resistant Microbial Communities Associated with Wastewater Treatment Alicia Sorgen 1, James Johnson 2, Kevin Lambirth 2, Sandra M. Clinton 3 , Molly Redmond 1 , Anthony Fodor 2 and Cynthia Gibas 2,* 1 Department of Biological Sciences, University of North Carolina at Charlotte, Charlotte, NC 28223, USA; [email protected] (A.S.); [email protected] (M.R.) 2 Department of Bioinformatics and Genomics, University of North Carolina at Charlotte, Charlotte, NC 28223, USA; [email protected] (J.J.); [email protected] (K.L.); [email protected] (A.F.) 3 Department of Geography & Earth Sciences, University of North Carolina at Charlotte, Charlotte, NC 28223, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-704-687-8378 Abstract: Bacterial resistance to antibiotics is a growing global concern, threatening human and environmental health, particularly among urban populations. Wastewater treatment plants (WWTPs) are thought to be “hotspots” for antibiotic resistance dissemination. The conditions of WWTPs, in conjunction with the persistence of commonly used antibiotics, may favor the selection and transfer of resistance genes among bacterial populations. WWTPs provide an important ecological niche to examine the spread of antibiotic resistance. We used heterotrophic plate count methods to identify Citation: Sorgen, A.; Johnson, J.; phenotypically resistant cultivable portions of these bacterial communities and characterized the Lambirth, K.; Clinton, -

Characterization of the Dominant Bacterial Communities Associated with Terrestrial Isopod Species Based on 16S Rdna Analysis by PCR-DGGE
Open Journal of Ecology, 2018, 8, 495-509 http://www.scirp.org/journal/oje ISSN Online: 2162-1993 ISSN Print: 2162-1985 Characterization of the Dominant Bacterial Communities Associated with Terrestrial Isopod Species Based on 16S rDNA Analysis by PCR-DGGE Delhoumi Majed, Zaabar Wahiba, Bouslama Mohamed Fadhel, Achouri Mohamed Sghaier* Laboratory of Bio-Ecology and Evolutionary Systematics, Department of Biology, Faculty of Sciences of Tunis, University of Tunis El Manar, Tunis, Tunisia How to cite this paper: Majed, D., Wahi- Abstract ba, Z., Fadhel, B.M. and Sghaier, A.M. (2018) Characterization of the Dominant Bacterial From the marine environment, woodlice gradually colonized terrestrial areas Communities Associated with Terrestrial benefiting from the symbiotic relationship with the bacterial community that Isopod Species Based on 16S rDNA Analy- they host. Indeed, they constitute the only group of Oniscidea suborder that sis by PCR-DGGE. Open Journal of Ecolo- gy, 8, 495-509. has succeed to accomplish their lives in terrestrial even desert surfaces. Here- https://doi.org/10.4236/oje.2018.89030 in they play an important role in the dynamic of ecosystems and the decom- position of litter. So to enhance our understanding of the sea-land transition Received: January 30, 2018 and other process like decomposition and digestion of detritus, we studied Accepted: September 15, 2018 Published: September 18, 2018 the bacterial community associated with 11 specimens of terrestrial isopods belonging to six species using a Culture independent approach (DGGE). Copyright © 2018 by authors and Bands sequencing showed that the cosmopolitan species Porcellionides prui- Scientific Research Publishing Inc. nosus has the most microbial diversity. -

Ochrobactrum Rhizosphaerae Sp. Nov. and Ochrobactrum Thiophenivorans Sp
International Journal of Systematic and Evolutionary Microbiology (2008), 58, 1426–1431 DOI 10.1099/ijs.0.65407-0 Ochrobactrum rhizosphaerae sp. nov. and Ochrobactrum thiophenivorans sp. nov., isolated from the environment Peter Ka¨mpfer,1 Angela Sessitsch,2 Michael Schloter,3 Birgit Huber,4 Hans-Ju¨rgen Busse4 and Holger C. Scholz5 Correspondence 1Institut fu¨r Angewandte Mikrobiologie, Justus-Liebig-Universita¨t Giessen, D-35392 Giessen, Peter Ka¨mpfer Germany peter.kaempfer@umwelt. 2Austrian Research Centers GmbH, Department of Bioresources, A-2444 Seibersdorf, Austria uni-giessen.de 3Helmholtz Zentrum Mu¨nchen, German Research Center for Environmental Health, Terrestrial Ecogenetics, Ingolstaedter Landstrasse 1, D-85764 Neuherberg, Germany 4Institut fu¨r Bakteriologie, Mykologie und Hygiene, Veterina¨rmedizinische Universita¨t Wien, A-1210 Wien, Austria 5Bundeswehr Institute of Microbiology, D-80937 Munich, Germany Two Gram-negative, rod-shaped, non-spore-forming bacteria, PR17T and DSM 7216T, isolated from the potato rhizosphere and an industrial environment, respectively, were studied for their taxonomic allocation. By rrs (16S rRNA) gene sequencing, these strains were shown to belong to the Alphaproteobacteria, most closely related to Ochrobactrum pseudogrignonense (98.4 and 99.3 % similarity to the type strain, respectively). Chemotaxonomic data (major ubiquinone Q-10; major polyamines spermidine, sym-homospermidine and putrescine; major polar lipids phosphatidylethanolamine, phosphatidylmonomethylethanolamine, phosphatidylglycerol and phosphatidylcholine and the Ochrobactrum-specific unidentified aminolipid AL2; major fatty acids C18 : 1v7c and C19 : 0 cyclo v8c) supported the genus affiliation. The results of DNA–DNA hybridization and physiological and biochemical tests allowed genotypic and phenotypic differentiation of the isolates from all hitherto-described Ochrobactrum species. Hence, both isolates represent novel species of the genus Ochrobactrum, for which the names Ochrobactrum rhizosphaerae sp. -

Brucellosis 2014 International Research Conference
SCHEDULE 2014 Federal Institute for Risk Assessment Brucellosis 2014 International Research Conference BERLIN | 9–12 September 2014 Imprint Abstracts Brucellosis 2014 International Research Conference All authors are responsible for the content of their respective abstracts. Federal Institute for Risk Assessment Communication and Public Relations Office Max-Dohrn-Straße 8–10 10589 Berlin Germany Berlin 2014 222 Pages Photo: flobox/Quelle: PHOTOCASE Printing: cover, content pages and bookbinding BfR-printing house Brucellosis 2014 International Research Conference 3 Welcoming Addresses Andreas Hensel President of the Federal Institute for Risk Assessment (BfR) Welcoming address by the President of the Federal Institute for Risk Assessment More than 100 years after the first description of Micrococcus melitensis by Sir David Bruce, brucellosis is still of major public health concern both in endemic and non-endemic countries all over the world. The impact on animal and human health is tremendous and eradication and control of this zoonotic disease remains a global and interdisciplinary challenge. Alt- hough eradicated in German livestock brucellosis is still a matter of interest because it has emerged as a disease among immigrants associated with diagnostic delays, possibly result- ing in treatment failures, relapses, chronic courses, focal complications, and a high case- fatality rate. The identification of health risks is the guiding principle for our work in the field of food safety and consumer protection. A total of 800 employees spare no effort to prove that no risk is more fun . I am delighted to host the Brucellosis 2014 International Research Conference at the Federal Institute for Risk Assessment, here in Berlin, the capital city of Germany, which was home and domain of so many Nobel laureates. -

Product Information Sheet for NR-50276
Product Information Sheet for NR-50276 Brucella melitensis, Strain 16MΔvjbR abortion and infertility. Transmission from animal to human via contact with infected animal products or through the air may lead to Malta (or undulant) fever, a long debilitating Catalog No. NR-50276 disease treatable by a prolonged course of antibiotics. This reagent is the property of the U.S. Government. Brucella species are recognized as potential agricultural, civilian, and military bioterrorism agents.4 For research use only. Not for human use. Material Provided: Contributor: Each vial contains approximately 0.7 mL of bacterial culture Thomas A. Ficht, Professor, College of Veterinary Medicine in Tryptic Soy broth supplemented with 10% glycerol. and Biological Sciences, Department of Veterinary Pathobiology, Texas A&M University, College Station, Texas, Note: If homogeneity is required for your intended use, please USA purify prior to initiating work. Manufacturer: Packaging/Storage: BEI Resources NR-50276 was packaged aseptically, in screw-capped plastic cryovials. The product is provided frozen and should be Product Description: stored at -60°C or colder immediately upon arrival. For long- Bacteria Classification: Brucellaceae, Brucella term storage, the vapor phase of a liquid nitrogen freezer is Species: Brucella melitensis recommended. Freeze-thaw cycles should be avoided. Biotype/Biovar: 1 Strain: 16MΔvjbR Growth Conditions: Original Source: Brucella melitensis (B. melitensis), strain Note: Passage of this organism can result in the accumulation 16MΔvjbR -

A Small Non-Coding RNA Facilitates Brucella Melitensis Intracellular Survival by Regulating the Expression of Virulence Factor T
International Journal of Medical Microbiology 309 (2019) 225–231 Contents lists available at ScienceDirect International Journal of Medical Microbiology journal homepage: www.elsevier.com/locate/ijmm A small non-coding RNA facilitates Brucella melitensis intracellular survival by regulating the expression of virulence factor T Yufei Wanga,1, Yuehua Keb,1, Cuijuan Duana,1, Xueping Maa, Qinfang Haoa, Lijie Songa, ⁎⁎⁎ Xiaojin Guoa, Tao Suna, Wei Zhanga, Jing Zhanga, Yiwen Zhaoa, Zhijun Zhongc, , ⁎⁎ ⁎ Xiaoli Yanga, , Zeliang Chenb,d, a Department of laboratory medicine, The Third Medical Center, General Hospital of the Chinese People’s Liberation Army, Beijing 100039, China b Department of Infectious Disease Control, Center of Disease Control and Prevention, Beijing 100071, China c Key Laboratory of Animal Disease and Human Health of Sichuan Province, College of Veterinary Medicine, Sichuan Agricultural University, Sichuan 611130, China d Key Laboratory of Livestock Infectious Diseases in Northeast China, Ministry of Education, Key Laboratory of Zoonosis of Liaoning Province, College of Aninal Science and Veterinary Medicine, Shenyang Agricultural University, Liaoning, 110866, China ARTICLE INFO ABSTRACT Keywords: Brucella species are the causative agents of brucellosis, a worldwide zoonotic disease that affects a broad range of Brucella mammals and causes great economic losses. Small regulatory RNAs (sRNAs) are post-transcriptional regulatory sRNA molecules that participate in the stress adaptation and pathogenesis of Brucella. In this study, we characterized Intracellular survival the role of a novel sRNA, BSR1141, in the intracellular survival and virulence of Brucella melitensis. The results Stress response show that BSR1141 was highly induced during host infections and under in vitro stress situations that simulated Virulence the conditions encountered within host phagocytes. -
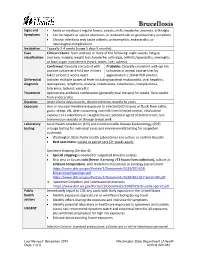
Brucellosis Reporting and Investigation Guideline
Brucellosis Signs and • Acute or insidious irregular fevers, sweats, chills, headache, anorexia, arthralgia Symptoms • Can be hepatic or splenic abscesses, or osteoarticular or genitourinary symptoms • Chronic infections may cause arthritis, osteomyelitis, endocarditis, or neurological complications Incubation Typically 2-4 weeks (range 5 days-5 months) Case Clinical criteria: fever and one or more of the following: night sweats, fatigue, classification anorexia, myalgia, weight loss, headache, arthralgia, arthritis/spondylitis, meningitis, or focal organ involvement (heart, testes, liver, spleen) Confirmed: Clinically consistent with Probable: Clinically consistent with epi link positive culture or 4-fold rise in titers to human or animal case or titer by taken at least 2 weeks apart agglutination ≥ 160 or PCR positive Differential Includes multiple causes of fever including bacterial endocarditis, viral hepatitis, diagnosis leptospirosis, lymphoma, malaria, rickettsioses, tuberculosis, toxoplasmosis, tularemia, typhoid, vasculitis Treatment Appropriate antibiotic combination (generally dual therapy) for weeks. Rare deaths from endocarditis. Duration Acute illness days to week, chronic infection months to years Exposure Skin or mucosal membrane exposure to infected birth tissues or fluids from cattle, goats, sheep, elk, deer; consuming raw milk from infected animal; inhalational exposure in a laboratory or slaughterhouse; potential agent of bioterrorism; rare transmission sexually or through breast milk Laboratory Local Health Jurisdiction -

Brucellosis Annual Report 2018
Brucellosis Annual Report 2018 Brucellosis Brucellosis is a Class A Disease. It must be reported to the state within 24 hours by calling the number listed on the website. Brucellosis is a zoonotic infection of domesticated and wild animals, caused by bacteria of the genus Brucella. Humans become infected by ingestion of food products of animal origin (such as undercooked meat or unpasteurized milk or dairy products), direct contact with infected animals, or inhalation of infectious aerosols. Brucella abortus (cattle), B.melitensis (sheep and goats), B.suis (pigs), and B.canis (dogs), are the most common species. The most common etiology in the U.S. is B.melitensis. Marine Brucella (B.ceti and B.pinnipedialis) may also pose a risk to humans who interact with marine animals; people should avoid contact with stranded or dead marine mammals. Infection may cause a range of symptoms, including fever, sweats, malaise, anorexia, headache, joint and muscle pain, and fatigue. Some symptoms may last for prolonged periods of time including recurrent fevers, arthritis, swelling of the testicle and scrotum area, swelling of the heart, swelling of the liver and/or spleen, neurologic symptoms, chronic fatigue, and depression. Treatment consists of antibiotics, but recovery may take a few weeks to several months. Bovine brucellosis caused by B. abortus, is a bacterial infection transmitted through oral exposure to uterine discharges from infected cows at time of calving or abortion. This previously common disease has been eliminated from the state through the cooperation of the cattle industry and state-federal animal health officials. On November 1, 2000, Louisiana was declared free of brucellosis in cattle. -

Culturable Aerobic and Facultative Bacteria from the Gut of the Polyphagic Dung Beetle Thorectes Lusitanicus
Insect Science (2015) 22, 178–190, DOI 10.1111/1744-7917.12094 ORIGINAL ARTICLE Culturable aerobic and facultative bacteria from the gut of the polyphagic dung beetle Thorectes lusitanicus Noemi Hernandez´ 1,Jose´ A. Escudero1, Alvaro´ San Millan´ 1, Bruno Gonzalez-Zorn´ 1, Jorge M. Lobo2,Jose´ R. Verdu´ 3 and Monica´ Suarez´ 1 1Department Sanidad Animal, Facultad de Veterinaria, Universidad Complutense de Madrid, Avenida Puerta de Hierro s/n, Madrid, CP 28040, 2Department Biogeograf´ıa y Cambio Global, Museo Nacional de Ciencias Naturales, CSIC, JoseGuti´ errez´ Abascal 2, Madrid 28006, and 3I.U.I. CIBIO (Centro Iberoamericano de la Biodiversidad), Universidad de Alicante, Carretera de San Vicente del Raspeig s/n, Alicante 03080, Spain Abstract Unlike other dung beetles, the Iberian geotrupid, Thorectes lusitanicus, exhibits polyphagous behavior; for example, it is able to eat acorns, fungi, fruits, and carrion in addition to the dung of different mammals. This adaptation to digest a wider diet has physiological and developmental advantages and requires key changes in the composition and diversity of the beetle’s gut microbiota. In this study, we isolated aerobic, facultative anaerobic, and aerotolerant microbiota amenable to grow in culture from the gut contents of T. lusitanicus and resolved isolate identity to the species level by sequencing 16S rRNA gene fragments. Using BLAST similarity searches and maximum likelihood phylogenetic analyses, we were able to reveal that the analyzed fraction (culturable, aerobic, facultative anaerobic, and aerotolerant) of beetle gut microbiota is dominated by the phyla Pro- teobacteria, Firmicutes,andActinobacteria. Among Proteobacteria, members of the order Enterobacteriales (Gammaproteobacteria) were the most abundant.