Brucellosis History Summary by Russell W
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Compendium of Veterinary Standard Precautions for Zoonotic Disease Prevention in Veterinary Personnel
Compendium of Veterinary Standard Precautions for Zoonotic Disease Prevention in Veterinary Personnel National Association of State Public Health Veterinarians Veterinary Infection Control Committee 2010 Preface.............................................................................................................................................................. 1405 I. INTRODUCTION................................................................................................................................... 1405 A. OBJECTIVES...................................................................................................................................... 1405 B. BACKGROUND................................................................................................................................. 1405 C. CONSIDERATIONS.......................................................................................................................... 1405 II. ZOONOTIC DISEASE TRANSMISSION................................................................................................ 1406 A. SOURCE ............................................................................................................................................ 1406 B. HOST SUSCEPTIBILITY.................................................................................................................... 1406 C. ROUTES OF TRANSMISSION........................................................................................................... 1406 1. CONTACT TRANSMISSION......................................................................................................... -

Herd Health Protocols for Dromedary Camels (Camelus Dromedarius) at Mpala Ranch and Research Centre, Laikipia County, Kenya
Herd Health Protocols for Dromedary Camels (Camelus dromedarius) at Mpala Ranch and Research Centre, Laikipia County, Kenya September 23rd, 2012 Andrew Springer Browne, MVB Veterinary Public Health Program University of Missouri – Columbia, USA Sharon Deem, DVM, PhD, DACZM Institute for Conservation Medicine Saint Louis Zoo, St. Louis, MO, USA This guide was made to solidify husbandry and record protocols as well as give feasible advice for common health problems in camels at the Mpala Ranch and Research Centre. For thorough reviews of camel diseases and health, see “A Field Manual of Camel Diseases” by Kohler-Rollefson and “Medicine and Surgery of Camelids” by Fowler. Acknowledgments Many thanks to Margaret Kinnaird and Mike Littlewood for their patience and support during our visit. Thank you to Laura Budd and Sina Mahs for their incredible help and time dedicated to the project. Finally, a special thanks to S. Moso, Eputh, Abduraman, Adow, Abdulai, Ekomoel, and Ewoi for working and living with the camels every day. Asante sana. - Springer and Sharon ii Table of Contents Page Key Management Goals 1 Record Keeping 2 Electronic and Paper Records 3 Entering Data into Excel Database 4 Excel Database Legend 5 Husbandry 6 Calf Care 7 Maternal Rejection 8 Dam Care 9 Branding and Identification 10 Weight Estimates/Body Condition Scoring 11 Milking Schedule and Boma Rotation 12 Veterinary Care 13 Preventive Veterinary Health Care 14 Diagnostics 15 Abortion 16 Skin Wounds 17 Abscess Treatment 18 Mastitis 19 Eye Problems 20 Diseases of Special -

Genital Brucella Suis Biovar 2 Infection of Wild Boar (Sus Scrofa) Hunted in Tuscany (Italy)
microorganisms Article Genital Brucella suis Biovar 2 Infection of Wild Boar (Sus scrofa) Hunted in Tuscany (Italy) Giovanni Cilia * , Filippo Fratini , Barbara Turchi, Marta Angelini, Domenico Cerri and Fabrizio Bertelloni Department of Veterinary Science, University of Pisa, Viale delle Piagge 2, 56124 Pisa, Italy; fi[email protected] (F.F.); [email protected] (B.T.); [email protected] (M.A.); [email protected] (D.C.); [email protected] (F.B.) * Correspondence: [email protected] Abstract: Brucellosis is a zoonosis caused by different Brucella species. Wild boar (Sus scrofa) could be infected by some species and represents an important reservoir, especially for B. suis biovar 2. This study aimed to investigate the prevalence of Brucella spp. by serological and molecular assays in wild boar hunted in Tuscany (Italy) during two hunting seasons. From 287 animals, sera, lymph nodes, livers, spleens, and reproductive system organs were collected. Within sera, 16 (5.74%) were positive to both rose bengal test (RBT) and complement fixation test (CFT), with titres ranging from 1:4 to 1:16 (corresponding to 20 and 80 ICFTU/mL, respectively). Brucella spp. DNA was detected in four lymph nodes (1.40%), five epididymides (1.74%), and one fetus pool (2.22%). All positive PCR samples belonged to Brucella suis biovar 2. The results of this investigation confirmed that wild boar represents a host for B. suis biovar. 2 and plays an important role in the epidemiology of brucellosis in central Italy. Additionally, epididymis localization confirms the possible venereal transmission. Citation: Cilia, G.; Fratini, F.; Turchi, B.; Angelini, M.; Cerri, D.; Bertelloni, Keywords: Brucella suis biovar 2; wild boar; surveillance; epidemiology; reproductive system F. -

Brucellosis in the Kakheti Region of The
DISSERTATION A SYSTEMIC REVIEW OF BRUCELLOSIS IN THE KAKHETI REGION OF THE COUNTRY OF GEORGIA: AN EVALUATION OF THE DISEASE ECOLOGY, RISK FACTORS AND SUGGESTIONS FOR DISEASE CONTROL Submitted by Karyn Alicia Havas Department of Clinical Sciences In partial fulfillment of the requirements For the Degree of Doctor of Philosophy Colorado State University Fort Collins, Colorado Fall 2011 Doctoral Committee: Advisor: Mo D. Salman Ashley E. Hill Robert J. Callan Shana C. Gillette Ann L. Magennis Copyright by Karyn A. Havas 2011 All rights reserved ABSTRACT A SYSTEMIC REVIEW OF BRUCELLOSIS IN THE KAKHETI REGION OF THE COUNTRY OF GEORGIA: AN EVALUATION OF THE DISEASE ECOLOGY, RISK FACTORS AND SUGGESTIONS FOR DISEASE CONTROL Human brucellosis is a neglected disease of poverty often found in highly agrarian, livestock dependent societies (World Health Organization, 2006). It is a purely zoonotic disease in that animals infect humans but there is not human-to-human transmission (Corbel, 2006). The highest human incidence of brucellosis in the country of Georgia is in the eastern region of Kakheti (Navdarashvili et al., 2005), which is also home to the majority of the country’s sheep and a significant portion of the country’s cattle population (Kvinikadze et al., 2009). In humans, brucellosis is acquired from animals either through direct contact with infected and shedding animals or their afterbirth or via consumption of contaminated dairy products made from the raw milk of a shedding animal. In Georgia, B. melitensis is the predominant species cultured from ill humans and has been cultured from sheep as well (Malania et al., 2009; Onashvili et al., 2009). -

Integrated Mrna-Seq and Mirna-Seq Analysis of Goat fibroblasts Response to Brucella Melitensis Strain M5-90
Integrated mRNA-seq and miRNA-seq analysis of goat fibroblasts response to Brucella Melitensis strain M5-90 Baobao Li*, Si Chen*, Chengqiang Wang, Qiaoling Chen, Churiga Man, Qi An, Zhenxing Zhang, Zhiyong Liu, Li Du and Fengyang Wang Hainan Key Lab of Tropical Animal Reproduction, Breeding and Epidemic Disease Research, Animal Genetic Engineering Key Lab of Haikou, College of Animal Science and Technology, Hainan University, Hainan Key Lab of Tropical Animal Reproduction, Haikou, Hainan, China * These authors contributed equally to this work. ABSTRACT Brucellosis is a globally zoonotic bacterial disease of humans and various animals including goats, sheep, and cattle. Brucella melitensis M5-90, a live attenuated vaccine strain, has been widely used to prevent brucellosis in goats and sheep. However, the molecular mechanisms governing protective immunity response in non-professional phagocytes infected with B. melitensis M5-90 have not been fully investigated, especially in goats. In our research, goat fibroblasts were used as in vitro models to determine these mechanisms by transcriptome analysis. After incubating with B. melitensis M5-90 3 h, the infected goat fibroblasts were collected at 0 h, 4 h, 24 h, 48 h and 72 h for RNA-seq. The results indicated that there were totally 11,819 differentially expressed genes (DEGs) and 777 differentially expressed (DE) miRNAs found in experiment groups compared with the control groups (|log2 (Foldchange)|≥1, FDR<0.05). GO and KEGG enrichment analyses revealed that down-regulated genes were involved in the riboflavin metabolism and positive regulation of IL-8 secretion pathway. The up-regulated genes were mainly involved in adaptive immunity, including TNF signaling pathway, MAPK signaling pathway and JAK/STAT pathway. -

A Novel Evidence of Immunological and Molecular Detection of Brucella Species in Camels
Journal of Pharmaceutical Research International 32(45): 78-87, 2020; Article no.JPRI.64484 ISSN: 2456-9119 (Past name: British Journal of Pharmaceutical Research, Past ISSN: 2231-2919, NLM ID: 101631759) A Novel Evidence of Immunological and Molecular Detection of Brucella Species in Camels Kavitha Manivannan1, Malathi Ramasamy2* and Hanaa Ahmed3** 1Department of Genetics, Tharb Camel Hospital, Qatar. 2Department of Biotechnology, Government Arts and Science College, Kurumbalur, Perambalur, 621107, Tamilnadu, India. 3Animal Health Research Institute, Egypt. Authors’ contributions This work was carried out in collaboration between all authors. Author KM designed the study, managed the analyses, performed the statistical analysis, wrote the protocol, and wrote the first draft of the manuscript. Authors MR and HA designed and supervised the study, wrote the protocol, interpreted the data, and managed the literature searches. All authors read and approved the final manuscript. Article Information DOI: 10.9734/JPRI/2020/v32i4531095 Editor(s): (1) Dr. Begum Rokeya, Bangladesh University of Health Sciences, Bangladesh. Reviewers: (1) Abdul Basit, University of Okara, Pakistan. (2) Hari Mohan Saxena, Guru Angad Dev Veterinary and Animal Sciences University, India. Complete Peer review History: http://www.sdiarticle4.com/review-history/64484 Received 10 November 2020 Original Research Article Accepted 17 January 2021 Published 02 February 2021 ABSTRACT Objectives: Brucellosis is a zoonosis with severe complications for both humans and animals. In this work, we intended to examine the Brucella infection in dromedary camels in Qatar by using different analysis. Materials and Methods: A total of 203 samples of dromedary camels were randomly collected from the nearby farms in Qatar. Real-time PCR for the genus specific Brucella cell surface salt extractable bcsp31 kDa protein gene were performed on DNA extracted from camel samples. -

Brucella Melitensis with Immune Response Upon Secondary
Humoral Immunity and CD4+ Th1 Cells Are Both Necessary for a Fully Protective Immune Response upon Secondary Infection with Brucella melitensis This information is current as of September 25, 2021. Marie-Alice Vitry, Delphine Hanot Mambres, Carl De Trez, Shizuo Akira, Bernhard Ryffel, Jean-Jacques Letesson and Eric Muraille J Immunol 2014; 192:3740-3752; Prepublished online 19 March 2014; Downloaded from doi: 10.4049/jimmunol.1302561 http://www.jimmunol.org/content/192/8/3740 http://www.jimmunol.org/ Supplementary http://www.jimmunol.org/content/suppl/2014/03/19/jimmunol.130256 Material 1.DCSupplemental References This article cites 93 articles, 41 of which you can access for free at: http://www.jimmunol.org/content/192/8/3740.full#ref-list-1 Why The JI? Submit online. by guest on September 25, 2021 • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2014 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Humoral Immunity and CD4+ Th1 Cells Are Both Necessary for a Fully Protective Immune Response upon Secondary Infection with Brucella melitensis Marie-Alice Vitry,* Delphine Hanot Mambres,* Carl De Trez,† Shizuo Akira,‡ Bernhard Ryffel,x,{ Jean-Jacques Letesson,*,1 and Eric Muraille‖,1 Brucella spp are intracellular bacteria that cause brucellosis, one of the most common zoonoses in the world. -

A Small Non-Coding RNA Facilitates Brucella Melitensis Intracellular Survival by Regulating the Expression of Virulence Factor T
International Journal of Medical Microbiology 309 (2019) 225–231 Contents lists available at ScienceDirect International Journal of Medical Microbiology journal homepage: www.elsevier.com/locate/ijmm A small non-coding RNA facilitates Brucella melitensis intracellular survival by regulating the expression of virulence factor T Yufei Wanga,1, Yuehua Keb,1, Cuijuan Duana,1, Xueping Maa, Qinfang Haoa, Lijie Songa, ⁎⁎⁎ Xiaojin Guoa, Tao Suna, Wei Zhanga, Jing Zhanga, Yiwen Zhaoa, Zhijun Zhongc, , ⁎⁎ ⁎ Xiaoli Yanga, , Zeliang Chenb,d, a Department of laboratory medicine, The Third Medical Center, General Hospital of the Chinese People’s Liberation Army, Beijing 100039, China b Department of Infectious Disease Control, Center of Disease Control and Prevention, Beijing 100071, China c Key Laboratory of Animal Disease and Human Health of Sichuan Province, College of Veterinary Medicine, Sichuan Agricultural University, Sichuan 611130, China d Key Laboratory of Livestock Infectious Diseases in Northeast China, Ministry of Education, Key Laboratory of Zoonosis of Liaoning Province, College of Aninal Science and Veterinary Medicine, Shenyang Agricultural University, Liaoning, 110866, China ARTICLE INFO ABSTRACT Keywords: Brucella species are the causative agents of brucellosis, a worldwide zoonotic disease that affects a broad range of Brucella mammals and causes great economic losses. Small regulatory RNAs (sRNAs) are post-transcriptional regulatory sRNA molecules that participate in the stress adaptation and pathogenesis of Brucella. In this study, we characterized Intracellular survival the role of a novel sRNA, BSR1141, in the intracellular survival and virulence of Brucella melitensis. The results Stress response show that BSR1141 was highly induced during host infections and under in vitro stress situations that simulated Virulence the conditions encountered within host phagocytes. -

Caprine Brucellosis: a Historically Neglected Disease with Significant Impact on Public Health
REVIEW Caprine brucellosis: A historically neglected disease with significant impact on public health Carlos A. Rossetti1*, Angela M. Arenas-Gamboa2, EstefanõÂa Maurizio1 1 Instituto de PatobiologõÂa, CICVyA-CNIA, INTA. NicolaÂs Repetto y de Los Reseros s/n, Hurlingham, Buenos Aires, Argentina, 2 Department of Veterinary Pathobiology, College of Veterinary Medicine & Biomedical Sciences, Texas A&M University, College Station, Texas, United States of America * [email protected] a1111111111 a1111111111 a1111111111 Abstract a1111111111 a1111111111 Caprine brucellosis is a chronic infectious disease caused by the gram-negative cocci-bacil- lus Brucella melitensis. Middle- to late-term abortion, stillbirths, and the delivery of weak off- spring are the characteristic clinical signs of the disease that is associated with an extensive negative impact in a flock's productivity. B. melitensis is also the most virulent Brucella spe- cies for humans, responsible for a severely debilitating and disabling illness that results in OPEN ACCESS high morbidity with intermittent fever, chills, sweats, weakness, myalgia, abortion, osteoarti- Citation: Rossetti CA, Arenas-Gamboa AM, cular complications, endocarditis, depression, anorexia, and low mortality. Historical obser- Maurizio E (2017) Caprine brucellosis: A historically neglected disease with significant vations indicate that goats have been the hosts of B. melitensis for centuries; but around impact on public health. PLoS Negl Trop Dis 11(8): 1905, the Greek physician Themistokles Zammit -
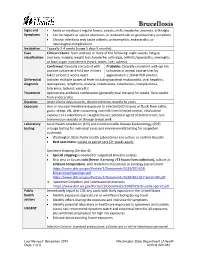
Brucellosis Reporting and Investigation Guideline
Brucellosis Signs and • Acute or insidious irregular fevers, sweats, chills, headache, anorexia, arthralgia Symptoms • Can be hepatic or splenic abscesses, or osteoarticular or genitourinary symptoms • Chronic infections may cause arthritis, osteomyelitis, endocarditis, or neurological complications Incubation Typically 2-4 weeks (range 5 days-5 months) Case Clinical criteria: fever and one or more of the following: night sweats, fatigue, classification anorexia, myalgia, weight loss, headache, arthralgia, arthritis/spondylitis, meningitis, or focal organ involvement (heart, testes, liver, spleen) Confirmed: Clinically consistent with Probable: Clinically consistent with epi link positive culture or 4-fold rise in titers to human or animal case or titer by taken at least 2 weeks apart agglutination ≥ 160 or PCR positive Differential Includes multiple causes of fever including bacterial endocarditis, viral hepatitis, diagnosis leptospirosis, lymphoma, malaria, rickettsioses, tuberculosis, toxoplasmosis, tularemia, typhoid, vasculitis Treatment Appropriate antibiotic combination (generally dual therapy) for weeks. Rare deaths from endocarditis. Duration Acute illness days to week, chronic infection months to years Exposure Skin or mucosal membrane exposure to infected birth tissues or fluids from cattle, goats, sheep, elk, deer; consuming raw milk from infected animal; inhalational exposure in a laboratory or slaughterhouse; potential agent of bioterrorism; rare transmission sexually or through breast milk Laboratory Local Health Jurisdiction -

Brucellosis Annual Report 2018
Brucellosis Annual Report 2018 Brucellosis Brucellosis is a Class A Disease. It must be reported to the state within 24 hours by calling the number listed on the website. Brucellosis is a zoonotic infection of domesticated and wild animals, caused by bacteria of the genus Brucella. Humans become infected by ingestion of food products of animal origin (such as undercooked meat or unpasteurized milk or dairy products), direct contact with infected animals, or inhalation of infectious aerosols. Brucella abortus (cattle), B.melitensis (sheep and goats), B.suis (pigs), and B.canis (dogs), are the most common species. The most common etiology in the U.S. is B.melitensis. Marine Brucella (B.ceti and B.pinnipedialis) may also pose a risk to humans who interact with marine animals; people should avoid contact with stranded or dead marine mammals. Infection may cause a range of symptoms, including fever, sweats, malaise, anorexia, headache, joint and muscle pain, and fatigue. Some symptoms may last for prolonged periods of time including recurrent fevers, arthritis, swelling of the testicle and scrotum area, swelling of the heart, swelling of the liver and/or spleen, neurologic symptoms, chronic fatigue, and depression. Treatment consists of antibiotics, but recovery may take a few weeks to several months. Bovine brucellosis caused by B. abortus, is a bacterial infection transmitted through oral exposure to uterine discharges from infected cows at time of calving or abortion. This previously common disease has been eliminated from the state through the cooperation of the cattle industry and state-federal animal health officials. On November 1, 2000, Louisiana was declared free of brucellosis in cattle. -

Brucella Melitensis: a Nasty Bug with Hidden Credentials for Virulence
Commentary Brucella melitensis: A nasty bug with hidden credentials for virulence Edgardo Moreno*† and Ignacio Moriyo´ n‡ *Tropical Disease Research Program, Veterinary School, National University, Apartado 304-3000, Heredia, Costa Rica; and ‡Department of Microbiology, University of Navarra, Apartado 177, 3208, Pamplona, Spain n September 23, 1905, a cargo carrying supporting information on the PNAS web secretion systems. On the other hand, O60 goats from Malta arrived in New site, www.pnas.org) and do not survive for some potential sequences for virulence York. The herd was kept in quarantine protracted periods of time outside the were discovered. For instance, Brucella because of several deaths that occurred dur- host. Their textbook description, ‘‘facul- recruits actin and activates small GTP- ing the journey. Crewmen, an agent from tative intracellular parasites,’’ does not ases during its internalization to cells (6), the U.S. Bureau of Animal Industry, which give credit to their true behavior, which is but the molecules involved in these was responsible for the shipment, and a better described as that of a facultatively events remain unknown. The revelation woman who drank milk that ‘‘escaped’’ from extracellular intracellular parasite. There- of putative genes coding for adhesins, the quarantine station displayed the char- fore, understanding the pathogenicity of invasins, and virG-like genes for attach- acteristic symptoms of ‘‘Mediterranean fe- brucellae is relevant not only because this ment and actin recruitment calls for the ver.’’ Lieutenant Colonel David Bruce, a pathogenicity represents a major infec- generation of null mutants in these se- physician of the Royal Army, who discov- tious disease but also because it will shed quences.