Brucellosis Annual Report 2018
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reportable Diseases and Conditions
KINGS COUNTY DEPARTMENT of PUBLIC HEALTH 330 CAMPUS DRIVE, HANFORD, CA 93230 REPORTABLE DISEASES AND CONDITIONS Title 17, California Code of Regulations, §2500, requires that known or suspected cases of any of the diseases or conditions listed below are to be reported to the local health jurisdiction within the specified time frame: REPORT IMMEDIATELY BY PHONE During Business Hours: (559) 852-2579 After Hours: (559) 852-2720 for Immediate Reportable Disease and Conditions Anthrax Escherichia coli: Shiga Toxin producing (STEC), Rabies (Specify Human or Animal) Botulism (Specify Infant, Foodborne, Wound, Other) including E. coli O157:H7 Scrombroid Fish Poisoning Brucellosis, Human Flavivirus Infection of Undetermined Species Shiga Toxin (Detected in Feces) Cholera Foodborne Disease (2 or More Cases) Smallpox (Variola) Ciguatera Fish Poisoning Hemolytic Uremic Syndrome Tularemia, human Dengue Virus Infection Influenza, Novel Strains, Human Viral Hemorrhagic Fever (Crimean-Congo, Ebola, Diphtheria Measles (Rubeola) Lassa, and Marburg Viruses) Domonic Acid Poisoning (Amnesic Shellfish Meningococcal Infections Yellow Fever Poisoning) Novel Virus Infection with Pandemic Potential Zika Virus Infection Paralytic Shellfish Poisoning Plague (Specify Human or Animal) Immediately report the occurrence of any unusual disease OR outbreaks of any disease. REPORT BY PHONE, FAX, MAIL WITHIN ONE (1) WORKING DAY Phone: (559) 852-2579 Fax: (559) 589-0482 Mail: 330 Campus Drive, Hanford 93230 Conditions may also be reported electronically via the California -

LIST of REPORTABLE COMMUNICABLE DISEASES in BC July 2009
LIST OF REPORTABLE COMMUNICABLE DISEASES IN BC July 2009 Schedule A: Reportable by all sources, including Laboratories Meningococcal Disease, All Invasive including “Primary Meningococcal Acquired Immune Deficiency Syndrome Pneumonia” and “Primary Meningococcal Anthrax Conjunctivitis” Botulism Mumps Brucellosis Neonatal Group B Streptococcal Infection Chancroid Paralytic Shellfish Poisoning (PSP) Cholera Pertussis (Whooping Cough) Congenital Infections: Plague Toxoplasmosis Poliomyelitis Rubella Rabies Cytomegalovirus Reye Syndrome Herpes Simplex Rubella Varicella-Zoster Severe Acute Respiratory Syndrome (SARS) Hepatitis B Virus Smallpox Listeriosis and any other congenital infection Streptococcus pneumoniae Infection, Invasive Creutzfeldt-Jacob Disease Syphilis Cryptococcal infection Tetanus Cryptosporidiosis Transfusion Transmitted Infection Cyclospora infection Tuberculosis Diffuse Lamellar Keratitis Tularemia Diphtheria: Typhoid Fever and Paratyphoid Fever Cases Waterborne Illness Carriers All causes Encephalitis: West Nile Virus Infection Post-infectious Yellow Fever Subacute sclerosing panencephalitis Vaccine-related Viral Schedule B: Reportable by Laboratories only Foodborne illness: All causes All specific bacterial and viral stool pathogens: Gastroenteritis epidemic: (i) Bacterial: Bacterial Campylobacter Parasitic Salmonella Viral Shigella Genital Chlamydia Infection Yersinia Giardiasis (ii) Viral Gonorrhea – all sites Amoebiasis Group A Streptococcal Disease, Invasive Borrelia burgdorferi infection H5 and H7 strains of the -

Genital Brucella Suis Biovar 2 Infection of Wild Boar (Sus Scrofa) Hunted in Tuscany (Italy)
microorganisms Article Genital Brucella suis Biovar 2 Infection of Wild Boar (Sus scrofa) Hunted in Tuscany (Italy) Giovanni Cilia * , Filippo Fratini , Barbara Turchi, Marta Angelini, Domenico Cerri and Fabrizio Bertelloni Department of Veterinary Science, University of Pisa, Viale delle Piagge 2, 56124 Pisa, Italy; fi[email protected] (F.F.); [email protected] (B.T.); [email protected] (M.A.); [email protected] (D.C.); [email protected] (F.B.) * Correspondence: [email protected] Abstract: Brucellosis is a zoonosis caused by different Brucella species. Wild boar (Sus scrofa) could be infected by some species and represents an important reservoir, especially for B. suis biovar 2. This study aimed to investigate the prevalence of Brucella spp. by serological and molecular assays in wild boar hunted in Tuscany (Italy) during two hunting seasons. From 287 animals, sera, lymph nodes, livers, spleens, and reproductive system organs were collected. Within sera, 16 (5.74%) were positive to both rose bengal test (RBT) and complement fixation test (CFT), with titres ranging from 1:4 to 1:16 (corresponding to 20 and 80 ICFTU/mL, respectively). Brucella spp. DNA was detected in four lymph nodes (1.40%), five epididymides (1.74%), and one fetus pool (2.22%). All positive PCR samples belonged to Brucella suis biovar 2. The results of this investigation confirmed that wild boar represents a host for B. suis biovar. 2 and plays an important role in the epidemiology of brucellosis in central Italy. Additionally, epididymis localization confirms the possible venereal transmission. Citation: Cilia, G.; Fratini, F.; Turchi, B.; Angelini, M.; Cerri, D.; Bertelloni, Keywords: Brucella suis biovar 2; wild boar; surveillance; epidemiology; reproductive system F. -

Veterinary Services Newsletter August 2017
August 2017 Veterinary Services Newsletter August 2017 Wildlife Health Laboratory Veterinary Services Staff NALHN Certification: The Wildlife Health Laboratory has been working towards joining the National Animal Health Laboratory Network (NALHN) in order to have ac- cess to all CWD testing kits. The commercial test kits for CWD are now restricted, and Branch Supervisor/Wildlife only NALHN approved laboratories have access to all the kits that are currently availa- Veterinarian: Dr. Mary ble. To be considered for the program, our laboratory must meet the ISO 17025 stand- Wood ards of quality control that assure our laboratory is consistently and reliably producing Laboratory Supervisor: accurate results. In addition, our laboratory will be regularly inspected by APHIS Veter- Hank Edwards inary Services, and we will be required to complete annual competency tests. Although we have been meeting most of the ISO standards for several years, applying to the Senior Lab Scientist: NAHLN has encouraged us to tighten many of our procedures and quality control moni- Hally Killion toring. We hope to have the application submitted by the middle of August and we have our first APHIS inspection in September. Senior Lab Scientist: Jessica Jennings-Gaines Brucellosis Lab Assistant: New CWD area for elk: The Wildlife Health Laboratory confirmed the first case of Kylie Sinclair CWD in an elk from hunt area 48. This animal was captured in elk hunt area 33 as part of the elk movement study in the Bighorns to study Brucellosis. She was found dead in Wildlife Disease Specialist: hunt area 48, near the very southeastern corner of Washakie County. -

AMD Projects: Deadly Disease Databases
CDC’s AMD Program AMD Projects Innovate • Transform • Protect CDC’s Advanced Molecular Detection (AMD) program fosters scientific innovation in genomic sequencing, epidemiology, and bioinformatics to transform public health and protect people from disease threats. AMD Project: Deadly Disease Databases Whole genome analysis and database development for anthrax (Bacillus anthracis), melioidosis (Burkholderia pseudomallei), and Brucellosis (Brucella spp.) Epidemiologists and forensic professionals can use whole genome sequencing – a way of determining an organism’s complete, detailed genome – and large databases to determine the source of dangerous germs. Having a large, accessible collection of disease pathogens could help scientists quickly find out if a certain illness is naturally occurring or the result of bioterrorism. CDC is establishing a public database where scientists from around the world can share information about these potentially deadly CDC is establishing public databases so that diseases. CDC scientists have begun sequencing the organisms that scientists from around the world can share information about deadly diseases like cause anthrax (Bacillus anthracis), brucellosis (Brucella spp.), and anthrax, brucellosis, and melioidosis. melioidosis (Burkholderia pseudomallei), three pathogens that could occur naturally or as the result of bioterrorism. Current methods of determining the genetic structure of these organisms are not standardized and sometimes not effective. Using whole genome sequencing for these pathogens will allow scientists www.cdc.gov/amd Updated: May 2017 to accurately and quickly find the geographic origin of the isolates and will improve overall knowledge and understanding of them. Having a detailed database of these genomes will also ensure quicker and more effective responses to outbreaks. For more information on anthrax, please visit www.cdc.gov/anthrax/index.html. -

A Small Non-Coding RNA Facilitates Brucella Melitensis Intracellular Survival by Regulating the Expression of Virulence Factor T
International Journal of Medical Microbiology 309 (2019) 225–231 Contents lists available at ScienceDirect International Journal of Medical Microbiology journal homepage: www.elsevier.com/locate/ijmm A small non-coding RNA facilitates Brucella melitensis intracellular survival by regulating the expression of virulence factor T Yufei Wanga,1, Yuehua Keb,1, Cuijuan Duana,1, Xueping Maa, Qinfang Haoa, Lijie Songa, ⁎⁎⁎ Xiaojin Guoa, Tao Suna, Wei Zhanga, Jing Zhanga, Yiwen Zhaoa, Zhijun Zhongc, , ⁎⁎ ⁎ Xiaoli Yanga, , Zeliang Chenb,d, a Department of laboratory medicine, The Third Medical Center, General Hospital of the Chinese People’s Liberation Army, Beijing 100039, China b Department of Infectious Disease Control, Center of Disease Control and Prevention, Beijing 100071, China c Key Laboratory of Animal Disease and Human Health of Sichuan Province, College of Veterinary Medicine, Sichuan Agricultural University, Sichuan 611130, China d Key Laboratory of Livestock Infectious Diseases in Northeast China, Ministry of Education, Key Laboratory of Zoonosis of Liaoning Province, College of Aninal Science and Veterinary Medicine, Shenyang Agricultural University, Liaoning, 110866, China ARTICLE INFO ABSTRACT Keywords: Brucella species are the causative agents of brucellosis, a worldwide zoonotic disease that affects a broad range of Brucella mammals and causes great economic losses. Small regulatory RNAs (sRNAs) are post-transcriptional regulatory sRNA molecules that participate in the stress adaptation and pathogenesis of Brucella. In this study, we characterized Intracellular survival the role of a novel sRNA, BSR1141, in the intracellular survival and virulence of Brucella melitensis. The results Stress response show that BSR1141 was highly induced during host infections and under in vitro stress situations that simulated Virulence the conditions encountered within host phagocytes. -

KDHE's Notifiable Disease List
REPORTABLE DISEASES IN KANSAS (K.S.A. 65-118, 65-128, 65-6001 - 65-6007, K.A.R. 28-1-2, 28-1-4, and 28-1-18. Changes effective as of 5/11/2018) For 4-hour reportable diseases report to the KDHE Epidemiology Hotline: 877-427-7317. For all other reportable diseases fax a Kansas Reportable Disease Form and any lab results to your local health department or to KDHE: 877-427-7318 within 24 hours or by the next business day. Acute flaccid myelitis Influenza, novel A virus infection Anthrax Legionellosis Anaplasmosis Listeriosis Arboviral disease, neuroinvasive and nonneuroinvasive Lyme disease (including chikungunya virus, dengue virus, La Malaria Crosse, West Nile virus, and Zika virus) Measles (rubeola) Babesiosis Meningococcal disease Blood lead levels (any results) Mumps Botulism Pertussis (whooping cough) Brucellosis Plague (Yersinia pestis) Campylobacteriosis Poliovirus Candida auris Psittacosis Carbapenem-resistant bacterial infection or Q Fever (Coxiella burnetii, acute and chronic) colonization Rabies, human Carbon monoxide poisoning Rabies, animal Chancroid Rubella Chickenpox (varicella) Salmonellosis, including typhoid fever Chlamydia trachomatis infection Severe Acute Respiratory Syndrome-associated Cholera coronavirus (SARS-CoV) Coccidioidomycosis Shiga toxin-producing Escherichia coli (STEC) Cryptosporidiosis Shigellosis Cyclosporiasis Smallpox Diphtheria Spotted fever rickettsiosis Ehrlichiosis Streptococcus pneumoniae, invasive disease Giardiasis Syphilis, all stages, including congenital syphilis Gonorrhea -
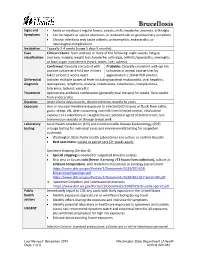
Brucellosis Reporting and Investigation Guideline
Brucellosis Signs and • Acute or insidious irregular fevers, sweats, chills, headache, anorexia, arthralgia Symptoms • Can be hepatic or splenic abscesses, or osteoarticular or genitourinary symptoms • Chronic infections may cause arthritis, osteomyelitis, endocarditis, or neurological complications Incubation Typically 2-4 weeks (range 5 days-5 months) Case Clinical criteria: fever and one or more of the following: night sweats, fatigue, classification anorexia, myalgia, weight loss, headache, arthralgia, arthritis/spondylitis, meningitis, or focal organ involvement (heart, testes, liver, spleen) Confirmed: Clinically consistent with Probable: Clinically consistent with epi link positive culture or 4-fold rise in titers to human or animal case or titer by taken at least 2 weeks apart agglutination ≥ 160 or PCR positive Differential Includes multiple causes of fever including bacterial endocarditis, viral hepatitis, diagnosis leptospirosis, lymphoma, malaria, rickettsioses, tuberculosis, toxoplasmosis, tularemia, typhoid, vasculitis Treatment Appropriate antibiotic combination (generally dual therapy) for weeks. Rare deaths from endocarditis. Duration Acute illness days to week, chronic infection months to years Exposure Skin or mucosal membrane exposure to infected birth tissues or fluids from cattle, goats, sheep, elk, deer; consuming raw milk from infected animal; inhalational exposure in a laboratory or slaughterhouse; potential agent of bioterrorism; rare transmission sexually or through breast milk Laboratory Local Health Jurisdiction -

Brucellosis History Summary by Russell W
Brucellosis History Summary by Russell W. Currier DVM, MPH Dr. Currier is a DVM, MPH, and Diplomate of the American College for Veterinary Preventive Medicine, who retired from a career as an Iowa State Public Health Veterinarian. Dr. Currier currently presides as President of the American Veterinary Medical History Society. Brucellosis is a sexually transmissible as well as contact-transmitted infection of livestock that can have adverse effects on the fetus and newborn. It occasionally transmits to humans in roles of animal handlers, raw milk consumers, and packing plant workers. In humans, the disease is infectious with long term chronic but distressing febrile episodes rarely fatal with modern clinical management; treatment remains in the domain of infectious disease physicians and should not be attempted by family doctors. The causative organism is Brucella melitensis [from sheep/goats], Brucella bovis [from cattle], Brucella suis [from swine] and Brucella canis [from dogs]. Historically, the disease was recognized in the Mediterranean region, particularly in goats and sheep, dating back to antiquity. Brucellosis-type illnesses were recognized by Hippocrates in his Epidemics writings; the Apostle Paul is considered to have been infected following his being shipwrecked on the Island of Malta and suffered from a recurrent illness or “thorn in my flesh” afterward. Britain maintained a military base on this island during the 18th and 19th centuries. During this period, several British physicians provided vivid descriptions of illness in garrisoned troops and physician David Bruce was dispatched to investigate; he isolated the causative organism from four fatal cases in 1887 and named it Micrococcus melitensis. -

Human Babesiosis and Ehrlichiosis Current Status
IgeneX_v1_A4_A4_2011 27/04/2012 17:26 Page 49 Tick-borne Infectious Disease Human Babesiosis and Ehrlichiosis – Current Status Jyotsna S Shah,1 Richard Horowitz2 and Nick S Harris3 1. Vice President, IGeneX Inc., California; 2. Medical Director, Hudson Valley Healing Arts Center, New York; 3. CEO and President, IGeneX Inc., California, US Abstract Lyme disease (LD), caused by the Borrelia burgdorferi complex, is the most frequently reported arthropod-borne infection in North America and Europe. The ticks that transmit LD also carry other pathogens. The two most common co-infections in patients with LD are babesiosis and ehrlichiosis. Human babesiosis is caused by protozoan parasites of the genus Babesia including Babesia microti, Babesia duncani, Babesia divergens, Babesia divergens-like (also known as Babesia MOI), Babesia EU1 and Babesia KO1. Ehrlichiosis includes human sennetsu ehrlichiosis (HSE), human granulocytic anaplasmosis (HGA), human monocytic ehrlichiosis (HME), human ewingii ehrlichiosis (HEE) and the recently discovered human ehrlichiosis Wisconsin–Minnesota (HWME). The resulting illnesses vary from asymptomatic to severe, leading to significant morbidity and mortality, particularly in immunocompromised patients. Clinical signs and symptoms are often non-specific and require the medical provider to have a high degree of suspicion of these infections in order to be recognised. In this article, the causative agents, geographical distribution, clinical findings, diagnosis and treatment protocols are discussed for both babesiosis and ehrlichiosis. Keywords Babesia, Ehrlichia, babesiosis, ehrlichiosis, human, Borrelia Disclosure: Jyotsna Shah and Nick Harris are employees of IGeneX. Richard Horowitz is an employee of Hudson Valley Healing Arts Center. Acknowledgements: The authors would like to thank Eddie Caoili, and Sohini Stone, for providing technical assistance. -

Fever, Malaise and Arthralgia: Brucellosis Or Salmonellosis in The
Original Investigation / Özgün Araştırma DOI: 10.5578/ced.202045 • J Pediatr Inf 2020;14(3):e106-e110 Fever, Malaise and Arthralgia: Brucellosis or Salmonellosis in the Differential Diagnosis in an Endemic Area Ateş, Halsizlik ve Artralji: Endemik Bir Bölgede Ayırıcı Tanıda Bruselloz ve Salmonelloz Başak Yıldız Atikan1(İD), Gülhadiye Avcu1(İD) 1 Clinic of Pediatric Infectious Diseases, Balikesir Ataturk City Hospital, Balikesir, Turkey Cite this article as: Yıldız Atikan B, Avcu G. Fever, malaise and arthralgia: brucellosis or salmonellosis in the differential diagnosis in an endemic area. J Pediatr Inf 2020;14(3):e106-e110. Abstract Öz Objective: Brucellosis and salmonellosis are both infectious, zoonotic Giriş: Bruselloz ve salmonelloz ülkemizde endemik olarak görülen en- and endemic diseases in Turkey. In this study, we aimed to report a group feksiyöz ve zoonotik hastalıklardır. Bu çalışmada hastanemize ateş, hal- of pediatric patients admitted to the hospital with fever, malaise and ar- sizlik ve eklem ağrısı yakınması ile başvuran ve bu iki hastalık açısından thralgia and diagnosed with either of the diseases. tetkik edilerek biri ile tanı almış pediatrik olgular geriye dönük olarak Material and Methods: We retrospectively analysed hospital records for incelenmesi amaçlanmıştır. gender, age, consumption of raw milk products, laboratory results, organ Gereç ve Yöntemler: Hastaların yaş, cinsiyet, çiğ süt ve süt ürünü tü- involvement, treatment choices and course of the disease. ketim öyküleri, klinik ve laboratuvar bulguları, organ tutuluşları, tedavi Results: Out of a total of 36 children, 30 were diagnosed with brucellosis uygulamaları ve prognozları retrospektif olarak değerlendirilerek sunul- and 6 with salmonellosis in two years. A total of 20 patients of 30 cases muştur. -

Report Communicable Diseases to the Local Health Department
Arizona Administrative Code Requires Providers to: Report Communicable Diseases to the Local Health Department *O Amebiasis Glanders O Respiratory disease in a health care institution or correctional facility Anaplasmosis Gonorrhea * Rubella (German measles) Anthrax Haemophilus influenzae, invasive disease Rubella syndrome, congenital Arboviral infection Hansen’s disease (Leprosy) *O Salmonellosis Babesiosis Hantavirus infection O Scabies Basidiobolomycosis Hemolytic uremic syndrome *O Shigellosis Botulism *O Hepatitis A Smallpox Brucellosis Hepatitis B and Hepatitis D Spotted fever rickettsiosis (e.g., Rocky Mountain spotted fever) *O Campylobacteriosis Hepatitis C Streptococcal group A infection, invasive disease Chagas infection and related disease *O Hepatitis E Streptococcal group B infection in an infant younger than 90 days of age, (American trypanosomiasis) invasive disease Chancroid HIV infection and related disease Streptococcus pneumoniae infection (pneumococcal invasive disease) Chikungunya Influenza-associated mortality in a child 1 Syphilis Chlamydia trachomatis infection Legionellosis (Legionnaires’ disease) *O Taeniasis * Cholera Leptospirosis Tetanus Coccidioidomycosis (Valley Fever) Listeriosis Toxic shock syndrome Colorado tick fever Lyme disease Trichinosis O Conjunctivitis, acute Lymphocytic choriomeningitis Tuberculosis, active disease Creutzfeldt-Jakob disease Malaria Tuberculosis latent infection in a child 5 years of age or younger (positive screening test result) *O Cryptosporidiosis